How Does Oxidative Phosphorylation Produce ATP?
Oxidative phosphorylation is the final stage of cellular respiration where ATP (adenosine triphosphate) is formed when electrons from NADH or FADH₂ pass through a chain of proteins and eventually reduce oxygen to water. The energy released during these electron transfers drives the production of ATP.
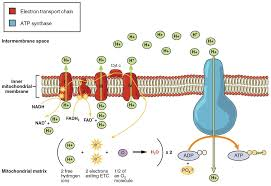
Where Oxidative Phosphorylation Occurs
In eukaryotes, oxidative phosphorylation occurs in the inner mitochondrial membrane. This membrane houses the electron transport chain (ETC) and ATP synthase, which work together to convert the energy of electrons into ATP.
Oxidative Phosphorylation Steps
Although the process may look complex, it can be broken down into four main oxidative phosphorylation steps:
Delivery of Electrons by NADH and FADH₂
NADH and FADH₂ donate their electrons to the initial components of the electron transport chain.
After donating electrons, NADH becomes NAD⁺ and FADH₂ becomes FAD, which can then be recycled back into other stages of cellular respiration, such as the [Krebs Cycle (TCA Cycle)].
Electron Transport and Proton Pumping
As electrons flow from one protein complex to another, energy is released.
This energy is used to pump protons (H⁺ ions) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient.
Splitting of Oxygen to Form Water
Oxygen (O₂) acts as the final electron acceptor.
After accepting the electrons, oxygen splits and combines with protons to form water (H₂O).
ATP Synthesis
The accumulated protons in the intermembrane space flow back into the matrix through the enzyme ATP synthase.
This flow of protons drives ATP synthase to phosphorylate ADP into ATP.
Chemiosmosis
Chemiosmosis is the underlying principle behind oxidative phosphorylation. The exergonic (energy-releasing) movement of electrons along the electron transport chain is coupled with the endergonic (energy-requiring) synthesis of ATP. The proton gradient formed across the inner mitochondrial membrane effectively links these two processes.
Electron Transport Chain (ETC)
The electron transport chain is a sequence of protein complexes and associated molecules that facilitate the stepwise transfer of electrons. Key points include:
It begins with electron donation by NADH or FADH₂.
Electrons move through several complexes, releasing energy at each step.
Protons are pumped into the intermembrane space, establishing a proton gradient.
The chain culminates with electrons combining with oxygen to form water.
This well-regulated release of energy ensures that cells harness maximum ATP production instead of losing energy as uncontrolled heat.
Oxidative Phosphorylation is Also Known As
Oxidative phosphorylation is also known as respiratory chain phosphorylation, as it primarily involves the respiratory chain in mitochondria.
Difference Between Substrate Level Phosphorylation and Oxidative Phosphorylation
Substrate level phosphorylation occurs when a phosphate group is directly transferred from a phosphorylated substrate to ADP, forming ATP. For instance, this happens during glycolysis or in the TCA cycle.
Oxidative phosphorylation, on the other hand, uses the electron transport chain and ATP synthase. Electrons from NADH or FADH₂ drive the synthesis of ATP via a proton gradient.
In simple terms, substrate level phosphorylation does not require an electron transport chain or oxygen, whereas oxidative phosphorylation relies on both.
Additional Insights
Significance: Oxidative phosphorylation generates the bulk of ATP in aerobic organisms, powering various metabolic and physiological processes.
Role in Health: Defects in components of the electron transport chain can lead to mitochondrial disorders, emphasising the importance of oxidative phosphorylation in normal cell function.
Prokaryotes: In prokaryotes, this process occurs in the plasma membrane since they lack mitochondria.
Practice Quiz on Oxidative Phosphorylation
Which molecule serves as the final electron acceptor in oxidative phosphorylation?
A) NAD⁺
B) Oxygen
C) FAD
D) ATP
Answer: B) Oxygen
Which enzyme synthesises ATP by utilising the proton gradient in the mitochondria?
A) Hexokinase
B) ATP synthase
C) Citrate synthase
D) Phosphofructokinase
Answer: B) ATP synthase
Oxidative phosphorylation occurs in the:
A) Cytosol
B) Outer mitochondrial membrane
C) Inner mitochondrial membrane
D) Nucleus
Answer: C) Inner mitochondrial membrane
Substrate level phosphorylation is different from oxidative phosphorylation because:
A) It directly converts ADP to ATP without an electron transport chain
B) It requires molecular oxygen
C) It occurs in mitochondria only
D) It produces water
Answer: A) It directly converts ADP to ATP without an electron transport chain
Related Topics


FAQs on Oxidative Phosphorylation: Process, Key Steps & Biological Importance
1. What is oxidative phosphorylation and where does it occur in a eukaryotic cell?
Oxidative phosphorylation is the primary metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate (ATP). It is the most efficient way for cells to generate energy. This entire process takes place on the inner mitochondrial membrane in eukaryotes.
2. What are the two major stages of oxidative phosphorylation?
Oxidative phosphorylation is comprised of two closely connected stages:
The Electron Transport Chain (ETC): High-energy electrons from NADH and FADH₂ are passed along a series of protein complexes, releasing energy. This energy is used to pump protons (H+) from the mitochondrial matrix into the intermembrane space.
Chemiosmosis: The accumulated protons create a steep electrochemical gradient (proton-motive force). Protons flow back into the matrix through an enzyme called ATP synthase, and the energy of this flow is used to convert ADP to ATP.
3. What are the final products of oxidative phosphorylation?
The key products of oxidative phosphorylation are a large amount of ATP (the cell's main energy currency) and water (formed when oxygen accepts the electrons). Additionally, the process regenerates NAD⁺ and FAD from NADH and FADH₂, which are essential for earlier stages of cellular respiration like glycolysis and the Krebs cycle to continue.
4. What is the role of the Electron Transport Chain (ETC) in oxidative phosphorylation?
The Electron Transport Chain's primary role is to act as an energy-conversion system. It harnesses the energy from electrons carried by NADH and FADH₂ and uses it to create a proton gradient. It does this by passing the electrons through a series of redox reactions, with each transfer releasing a small amount of energy used to pump protons across the inner mitochondrial membrane, setting the stage for ATP synthesis.
5. How does oxidative phosphorylation differ from substrate-level phosphorylation?
The main difference lies in the mechanism of ATP production. In oxidative phosphorylation, ATP is generated indirectly using the energy from a proton gradient established by the ETC. In contrast, substrate-level phosphorylation involves the direct transfer of a phosphate group from a high-energy substrate molecule to ADP, as seen in glycolysis and the Krebs cycle. Consequently, oxidative phosphorylation produces a much higher yield of ATP.
6. Why is oxygen's role as the final electron acceptor so critical for the entire process?
Oxygen is critical because it is the final destination for the electrons moving through the ETC. By accepting these electrons (and protons) to form water, oxygen keeps the chain flowing. Without oxygen, the electrons would have nowhere to go, causing the entire ETC to back up and halt. This would stop the pumping of protons, prevent the formation of the proton gradient, and shut down ATP production via ATP synthase.
7. How many ATP molecules are produced from one molecule of NADH and one of FADH₂?
On average, the oxidation of one molecule of NADH yields approximately 2.5 to 3 ATP molecules. The oxidation of one molecule of FADH₂ yields about 1.5 to 2 ATP molecules. The difference arises because NADH donates its electrons to Complex I of the ETC, while FADH₂ donates its electrons to Complex II, bypassing the first proton-pumping site.
8. What would happen if the inner mitochondrial membrane suddenly became leaky to protons?
If the inner mitochondrial membrane became permeable or 'leaky' to protons, the proton gradient established by the ETC would dissipate. Protons would flow back into the mitochondrial matrix without passing through the ATP synthase channel. This uncouples the ETC from ATP synthesis. The ETC would continue to function and consume oxygen, but since the proton-motive force is lost, very little or no ATP would be produced. The energy would instead be released as heat.
9. Explain the concept of 'chemiosmosis' in the context of oxidative phosphorylation.
Chemiosmosis is the process of using energy stored in a hydrogen ion (proton) gradient across a membrane to drive cellular work. In oxidative phosphorylation, it specifically describes how the proton-motive force (the high concentration of protons in the intermembrane space) powers ATP synthesis. As protons flow down their concentration gradient back into the matrix through the ATP synthase enzyme, they cause it to rotate, catalysing the phosphorylation of ADP to form ATP.
10. What is the overall biological importance of oxidative phosphorylation?
The biological importance is immense; it is the process that generates the vast majority (over 90%) of the ATP in aerobic organisms. This enormous and efficient energy supply is essential for powering nearly all cellular functions, including muscle contraction, nerve impulse transmission, DNA replication, and active transport across membranes. It is fundamental to sustaining the high energy demands of complex, multicellular life.










