Why Paper Chromatography is Essential for Studying Plant Pigments
A compound that absorbs light is called a pigment. Chlorophylls a and b are primary photosynthetic pigments that absorb light for photosynthesis. The accessory pigments carotenoids and xanthophyll absorb light and pass it to chlorophyll a. Even though chlorophyll is the primary pigment, the other pigments are essential to the plant's ability to produce colour and engage in photosynthesis because they absorb each light differently and effectively across the electromagnetic spectrum.
Paper Chromatography
Chromatography, which means "colour writing," is a Greek term that is formed from the words "chromo" and "graph". Chromatography enables the separation of the constituent parts of a given mixture, enabling scientists to observe and produce findings and theories.
Paper chromatography is a method for classifying dissolved substances according to how soluble they are in a given solvent, such as chlorophyll, carotene, and xanthophyll. Paper chromatography can be used to separate the colours in plant cells. The stationary element in chromatography paper permits the reaction between the solute and solvent to take place and produce results.
Leaf Chromatography
The separation of leaf colours using chromatography is known as leaf chromatography. Leaf chromatography is an experiment that is conducted to determine the colour of the photosynthetic pigments.
The experiment is conducted to learn about the pigments in the leaf, and it is mostly done by using paper and thin-layer chromatography. Let’s discuss some brief points of leaf chromatography.
There is a procedure by which this experiment is conducted in labs.
Sample leaves should be crushed into small pieces and put in a mortar for pestle grinding. Add solvent and keep using the pestle to crush.
Then, carefully draw a pencil line 1 cm from the bottom of the chromatography paper, spot a little amount of leaf extract repeatedly onto the centre of the line, and let each spot dry.
Make sure the paper dips into the solvent but the spot of leaf extract doesn't by suspending it using a pin attached to a bung within a test tube with a 1 cm depth of solvent.
The solvent is allowed to run up the paper until it is close to the bung, at which point the paper is removed. The solvent's location is marked, and the paper is allowed to dry.
The final chromatography paper is known as a chromatogram, and it may be photographed to determine the exact position of each pigment. Next, determine the Rf value for each pigment spot on the chromatogram.
The retention factor is pronounced Rf. The retention factor is calculated by dividing the component's travel distance by the solvent's travel distance.
The colour dissolves as the alcohol goes through the filter paper. Some pigments in the leaf travel more quickly than others because of their properties.
The pigment's movement rate is measured by the Rf (retention factor) value. Rf value = distance transported by pigment from origin to centre of pigment spot/distance from the origin to the solvent front. By applying this formula, you can determine the Rf value.
Separation of Plant Pigments by Paper Chromatography
The pigments in the plant's leaf are separated by paper chromatography, i.e., separation chromatography. It is the same as a leaf chromatography experiment. The process of paper chromatography is also the same as the leaf chromatography experiment.
Separation of Chlorophyll Pigments by Paper Chromatography
The chlorophyll molecule is present in the leaf and can be separated by using paper chromatography. The paper chromatography separates the pigments in the leaf based on the distance travelled by pigment molecules on the paper in a nonpolar solvent.
Separation of Plant Pigments by Paper Chromatography Diagram
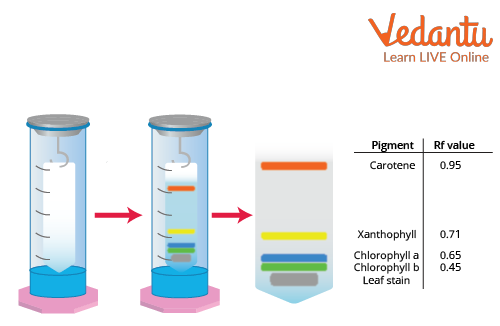
The Experimental Setup of Paper Chromatography
Chromatogram Report
The final chromatography paper is known as a chromatogram, and it may be photographed to determine the exact position of each pigment. The pattern of pigment spots on the chromatography paper at the conclusion of the experiment is called a chromatogram. Along with the alcohol, the pigments also migrate along the strips of paper.
Chromatography Conclusion
Carotene is identified as having the lowest molecular weight by its yellow to orange tint near the top of the paper. In the pigment separation of chlorophyll, chlorophyll may be distinguished by its blue or dark green hue. When chlorophyll pigments are separated, the colour yellow-light green identifies chlorophyll B. In the chromatography solvent, xanthophyll is more soluble since it has gone up the paper. This describes the conclusion of paper chromatography.
Conclusion
The pigments are light-absorbing molecules and are separated by using paper chromatography techniques in the lab. The pigments move on the paper based on their solubility in the solvent. Along with the alcohol, pigments also migrate along the strips of paper. Some pigments in the leaf travel more quickly than others because of their properties.


FAQs on Paper Chromatography: Separating Plant Pigments Made Simple
1. What is paper chromatography and how is it used to separate plant pigments?
Paper chromatography is a laboratory technique used to separate dissolved chemical substances by taking advantage of their different rates of migration across sheets of paper. For plant pigments, a spot of pigment extract is applied to a strip of chromatography paper (the stationary phase), which is then suspended in a jar with a solvent (the mobile phase). As the solvent moves up the paper, it dissolves the pigment mixture and carries it up the paper. Different pigments are separated based on their unique properties.
2. What is the main principle behind the separation of pigments in paper chromatography?
The separation of pigments is based on the principle of partition chromatography. The chromatography paper contains water trapped in it, which acts as the stationary phase. The solvent, which is the mobile phase, moves up the paper. Pigments are separated based on two key factors: their solubility in the solvent and their adsorption (attraction) to the paper. Pigments that are more soluble in the solvent and less strongly adsorbed to the paper travel further up, resulting in separation.
3. What are the main pigments found in a spinach leaf, and in what order do they appear on the chromatogram?
A typical chromatogram from a spinach leaf extract shows four distinct pigments. In order from the top (fastest moving) to the bottom (slowest moving), they are:
Carotenes: Appear yellow-orange.
Xanthophylls: Appear yellow.
Chlorophyll a: Appears bright or blue-green.
Chlorophyll b: Appears yellow-green.
4. What is an Rf value and what is its importance in chromatography?
The Retention Factor (Rf value) is a ratio calculated to identify a component in a mixture. It is defined as the ratio of the distance travelled by the pigment (solute) to the distance travelled by the solvent front. The formula is: Rf = Distance travelled by pigment / Distance travelled by solvent. Its importance lies in the fact that, under constant conditions (temperature, solvent, paper type), the Rf value for a specific compound is constant, which helps in its identification.
5. Why do different pigments like carotenes and chlorophylls travel at different rates up the paper?
The different travel rates are due to variations in their molecular structure, polarity, and solubility. Carotenes are highly soluble in the non-polar chromatography solvent and have a weak affinity for the polar paper, so they travel the farthest and fastest. In contrast, chlorophylls are more polar and adsorb more strongly to the polar cellulose paper, causing them to move slower. This differential movement results in their separation into distinct bands.
6. What common mistakes should a student avoid while performing the paper chromatography experiment?
To ensure accurate results, students should avoid these common mistakes:
Using a pen instead of a pencil: Ink contains pigments that would dissolve in the solvent and interfere with the results. A pencil mark is graphite, which is insoluble.
Letting the pigment spot touch the solvent: The initial spot must be above the solvent level so that it travels up the paper by capillary action, not dissolve directly into the solvent pool.
Not sealing the container: The chromatography jar must be sealed to create a saturated atmosphere, which prevents the solvent from evaporating off the paper as it rises.
7. How does the polarity of the solvent and the pigments influence the separation process?
The principle 'like dissolves like' is central here. The chromatography paper (cellulose) is a polar substance, while the solvent used (e.g., a mixture of petroleum ether and acetone) is largely non-polar. Pigments that are more non-polar, like carotenes, will have a greater affinity for the non-polar solvent and will be carried further up the paper. Pigments that are more polar, like chlorophylls, will be more attracted to the polar paper and will move shorter distances.
8. Can paper chromatography distinguish between chlorophyll a and chlorophyll b? If so, how?
Yes, paper chromatography can effectively distinguish between chlorophyll a and chlorophyll b. Although both are chlorophylls, chlorophyll b is more polar than chlorophyll a due to a slight difference in their molecular side groups. Because of its higher polarity, chlorophyll b adsorbs more strongly to the polar cellulose paper and is less soluble in the non-polar solvent. As a result, chlorophyll b travels slower and appears as a separate, lower band (yellow-green) compared to the higher band of chlorophyll a (blue-green).










