




Step-by-Step Method to Calculate Density in Class 9 Physics Experiments
Mass per unit volume is one method for describing a material's density. The lab can use a spring balance to determine the material's mass. The mass that was determined shall be referred to as weight per convention. If a solid has a regular geometric shape, its volume can be measured directly. Here, we will experiment to measure the density of a solid by measuring its mass and volume.
Table of Contents
Aim
Apparatus required
Theory
Result
Precautions
Aim
The aim of this experiment is to determine the density of a solid, which will be denser than water.
Apparatus Required
Spring balance, a beaker filled with water, a metal object, a measuring cylinder, string and a stand
Theory
The mass per unit volume of a substance is defined as the density of the substance. The formula for density is:
\[Density = \dfrac{{Mass}}{{Volume}}\].
S.I. unit of density is \[Kg/{m^3}\]. The ratio of a density of a substance to the ratio of the density of reference (water, in our case) is defined as the Relative density of the substance. The difference between density and relative density is that the density is mass divided by volume (its unit is ${kg/m^3}$). In contrast, relative density is the density of a substance divided by the density of reference (it has no units).
The density of water is 1000\[Kg/{m^3}\]. So, if the density of the solid is less than the water, then the solids will float on the water, and if the density of the solid is more than the water, i.e., the solid is denser, the solid will sink in water.
Spring balance is used to measure the weight of a substance, and weight can be defined as the force on an object due to the earth's gravity.
Force = mass x acceleration,
As weight is a force, so weight = mass x acceleration due to gravity,
Weight = m x g
Procedure
Tie the metal object with a string and attach the string to the hook of the spring balance. Take the reading of spring balance before attaching a metal object. Write it down as ‘x’. Make sure it’s zero. This is called zero error.
Tie the spring balance to the stand or hold it in hand. Measure the weight of the object. Consider this weight as ‘WF’.
Pour water into a given measuring cylinder. And note down its initial volume as ‘V1’.
Now put the metal object in a measuring cylinder which contains water. Do not let the object touch the sides of the cylinder or base. The water level rises after putting metal objects in the cylinder. Now note down the increased volume in the measuring cylinder. Write this volume as ‘V2’.
Take some more readings and perform the calculations to find the density of a given metal object.
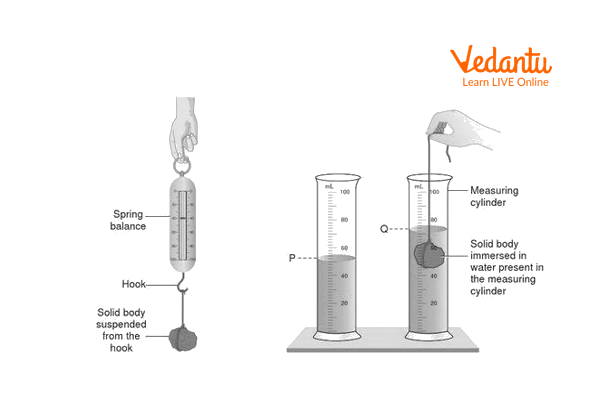
Density of Solid
Observations
An initial reading of spring balance x = 0
Weight of the metal object = W = WF – x
Mass of metal object = M = WF/9.8
Initial volume in measuring cylinder at P = V1
The final volume in the measuring cylinder at Q = V2
Volume of Metal object V = V2- V1
Calculations
${Density= {\dfrac{Mass (M)}{Volume(V)}}}$ = ……… (${kg/m^3}$)
Result
Density of Metal is ……………. (${kg/m^3}$)
Precautions
Make sure the spring balance reads zero initially.
Take all the readings of spring balance and measuring cylinder carefully such that your eyes are parallel to the reading.
Make sure that the metal object does not touch the sides of the cylinder.
Lab Manual Questions
1. Can you determine the density of any porous solid by using the spring balance and measuring cylinder setup?
Ans: We cannot find the density of porous solid using spring balance and measuring cylinder. Because the porous solid will absorb water, the mass of the solid will increase instead of decrease. This will affect the volume of water displaced.
2. How will the presence of air bubbles in the measuring cylinder affect the volume of the solid?
Ans: Air bubbles in liquid in the measuring cylinder will affect volume because air bubbles will occupy some space in the liquid, increasing the volume of the liquid.
3. What will happen to the density if we melt a metal cylinder and cast it in the shape of a cube? Give reason
Ans: Density will remain the same as the mass, and the volume of water displaced by the cylinder and cube will remain the same. So, the density will also remain the same.
4. At what temperature the density of water is maximum?
Ans: The density of water is maximum at 40C.
Viva Questions
1. Define density.
Ans: Density is mass divided by volume.
2. State SI unit of density.
Ans: SI unit of density is Kg/m3.
3. Define density and give its SI unit
Ans: We can define density as mass upon volume, SI unit is Kg/m3.
4. What is C.G.S unit of density?
Ans: C.G.S. unit of density is g/cm3.
5. What is relative density?
Ans: Relative density is the density of a substance divided by the density of reference.
6. What is the value of acceleration due to gravity?
Ans: 9.8m/s2
7. What is the difference in mass and weight?
Ans: Mass does not change with location but weight changes with a change in location.
8. What is the unit of relative density?
Ans: It is unitless.
9. What does spring balance measure?
Ans: Weight of body.
10. If we double the mass, what happens to density?
Ans: Density also doubles
Practice Based Questions
1. SI unit of Density is
N
g/cm3
kg/m3
m
Ans: c) kg/cm3
2. Formula of density is
${Mass \times Volume}$
${Mass/Volume}$
${Mass \times Weight}$
${Mass/Weight}$
Ans: b) ${Mass/Volume}$
3. Which of the following has the highest density?
Water
Ice
Oil
Iron
Ans: d) Iron
4. Relative density of water is
1000
100
10
1
Ans: d) 1
5. Two substances of relative densities d1 and d2 are mixed in the same volumes, then the relative density of the mixture becomes 4, and if mixed in the same masses, then the density of the mixture is 3. The values of d1 and d2 are _____ respectively
6, 2
4,2
12, 3
5, 6
Ans: a) 6, 2
6. What happens when we put ice in water?
Ice floats
Ice sinks
Ice floats partially and partially sinks
None of these
Ans: a) Ice floats
7. The body floats on water when its density is
More than water
Less than water
Equal to water
None of above
Ans: b) Less than water
8. If the mass of a body is halved
Density doubles
Density halves
Density remains same
None of these
Ans: b) Density halves
9. Density is
Directly proportional to mass
Directly proportional to volume
Inversely proportional to mass
Equal to Volume
Ans: a) Directly proportional to mass
10. Weight =
${m \times g}$
${m \times v}$
${v \times g}$
${v \times m}$
Ans: a) ${m \times g}$
Conclusion
In this experiment, we found the density value of a given solid using a spring balance and measuring cylinder. The density of a substance is mass divided by volume. When we put an object in water, the volume of water increases. This increase in volume is equal to the volume of the object.
FAQs on Explore Density of Solids: Practical Guide for Class 9 Physics (2025-26)
1. What are the most important types of questions to prepare for the Class 9 exam on the topic 'Density of a Solid'?
For your Class 9 exams, you should focus on a few key types of questions from this topic. Based on the CBSE 2025-26 pattern, expect:
- Practical-based questions: These require you to explain the procedure for finding the density of an irregular solid using a spring balance and measuring cylinder.
- Numerical problems: Simple calculations using the formula Density = Mass/Volume.
- Conceptual questions: Questions that test your understanding, such as why solids are denser than liquids.
- Application-based questions (HOTS): Scenarios involving concepts like sinking, floating, and relative density.
2. How do I find the density of an irregular solid using a spring balance and a measuring cylinder?
This is a very important practical-based question. Follow these steps for full marks:
- Step 1 (Find Mass): Use the spring balance to measure the mass of the solid in the air. Note the reading in grams (g).
- Step 2 (Find Volume): Fill a measuring cylinder with water to a certain level and note the initial volume (V1). Carefully immerse the solid in the water, ensuring it is fully submerged. Note the new water level (V2). The volume of the solid is the volume of water it displaces, which is (V2 - V1).
- Step 3 (Calculate Density): Use the formula Density = Mass / Volume. Divide the mass from Step 1 by the volume from Step 2 to get the density, usually in g/cm³.
3. What is the difference between mass and weight, and which one should be used for density calculations?
It's a crucial distinction for your exam. Mass is the amount of matter in an object and is constant everywhere, measured in kg or g. Weight is the force of gravity on that mass and can change depending on location, measured in Newtons (N). For calculating density, you must always use the mass of the object, not its weight.
4. Why does a small iron nail sink in water while a huge ship made of the same material floats?
This is a classic higher-order thinking question. An iron nail sinks because it is a solid block of iron, which is much denser than water. A ship, however, is not a solid block. It has a hollow structure that encloses a large volume of air. This makes the ship's average density (the total mass of the ship and the air inside, divided by its total volume) less than the density of water, allowing it to float.
5. How does heating a solid typically affect its density?
When you heat a solid, its particles gain energy and vibrate more, causing the solid to expand. This process is called thermal expansion. As the solid's volume increases but its mass remains the same, its overall density decreases. This is why a hot object is slightly less dense than the same object when it is cold.
6. What is relative density, and why is it considered important?
Relative density is a comparison of a substance's density to the density of water. It is calculated by the formula: Relative Density = Density of Substance / Density of Water. It is important because:
- It is a pure number with no units, which makes it easy to compare different materials.
- It immediately tells you if a substance will sink or float in water. If the relative density is greater than 1, it will sink. If it is less than 1, it will float.
7. What are some common mistakes to avoid in density experiments and calculations to score full marks?
To ensure you don't lose marks, watch out for these common errors:
- Parallax Error: Not reading the water level in the measuring cylinder at eye level.
- Unit Mismatch: Using mass in kilograms but volume in cubic centimetres without converting. Always use consistent units like g and cm³ or kg and m³.
- Incomplete Submersion: Not ensuring the solid is fully underwater when measuring its volume.
- Confusing Mass and Weight: Using the value for weight (in Newtons) instead of mass (in grams or kg) in the density formula.




































