




An Overview of Class 11 Biology Separation Of Plant Pigments Through Paper Chromatography Experiment
A plant contains several pigments that enable its metabolic capabilities. Chlorophyll, of course, is the most important plant pigment in the leaves of photosynthetic plants. It is what gives most plants their characteristic green colour and mediates the processes of photosynthesis as well. Besides chlorophyll, plants contain a group of pigments called carotenoids, yellow-orange pigments. Xanthophyll and carotene are the two most important carotenoids. How does one separate these pigments of a plant from visualising their colour? Carry out this simple yet interesting experiment to find out!
Table of Content
Aim
Apparatus required
Theory
Procedure
Observations
Result
Precautions
Aim
To separate and visualise plant pigments present in spinach leaf using the technique of paper chromatography
Apparatus Required
Spinach leaves, pestle and mortar, water, chromatography jar, ether acetone solvent, scissors, Whatman No.1 filter paper strips, watch glass, capillary tube, pencil, scale
Theory
A typical photosynthetic plant contains several pigments across its stems, leaves and flowers.
The leaves of a photosynthetic plant contain the photosynthetic pigments chlorophyll a and chlorophyll b, which impart green colour to the plant, and are contained in the chloroplasts.
They form the reaction centres of photosynthesis by absorbing the incident sunlight.
The second most commonly occurring plant pigments are the carotenoids- namely, xanthophyll and carotene. The carotenoids are the yellow-orange pigments of the plant, present within chromoplasts.
The different pigments occurring in the leaves of a plant can be visualised using paper chromatography.
Principles of Paper Chromatography
Martin and Synge developed paper chromatography in the year 1943. It is a simple technique used to visualise different components within a mixture.
Paper chromatography is based on the different solubilities of the various components of a mixture in a particular solvent. Based on their solubilities in the solvent, each component is separated.
Paper chromatography comprises two phases- the stationary phase, which is a nonpolar medium of cellulose and water, and the mobile phase, which is a polar, organic solvent (e.g. ether acetone solvent)
The mixture is loaded onto the paper, which is then suspended in an appropriate solvent (the mobile phase)
Absorption, solubility and capillarity are the three factors that affect the separation of the components and the distance travelled by each component from the initial spot.
Absorption holds the substance onto the paper. Capillary action pulls up the substances through the paper. Different solubilities separate the components in the mixture.
The most soluble and lighter substance moves farthest, while the least soluble and heavier substance separates early on near the loading point on the paper
The separated pigments appear as streaks at different positions on the chromatography paper.
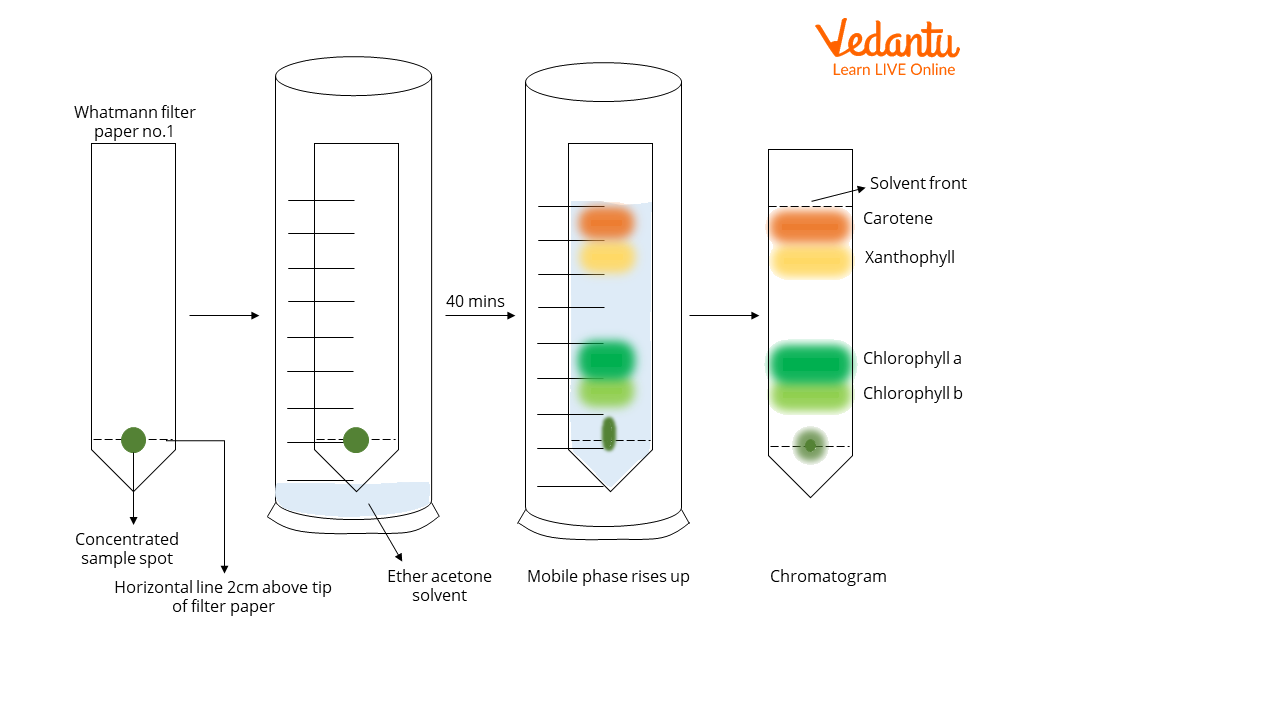
Procedure
Take a few fresh spinach leaves and wash them under distilled water
Using your scissors, cut the spinach leaves into small pieces and add them into the mortar, along with a few drops of acetone (~5mL). Grind into a fine paste
Take a strip of Whatman filter paper No.1 (6” x 1.5”) and cut one end such that it has a tapering (arrowed) notch
Using a pencil, mark a horizontal line 2 cm above the tip of the notch
Add just one drop of the prepared leaf paste onto the centre of this horizontal line using a capillary tube
Once the drop dries, add another drop of the sample onto the same spot
Repeat steps 5-6 at least 4-5 times. This will ensure a concentrated sample.
Add ~5mL of the ether acetone solvent into the chromatography jar
Now suspend the loaded filter paper onto the hook of the cork of the chromatography jar. For this step, one can also use a measuring cylinder, a cork, and a pushpin.
The pointed end of the paper should just touch the solvent once suspended. The horizontal line marked on the paper MUST NOT TOUCH the solvent
Allow the solvent to rise through the filter paper. This may take up to 40 minutes.
Once the pigments have separated into four distinct bands, and the solvent has stopped rising, remove the paper from the hook.
Mark the edge of the solvent on the paper. This is the solvent front.
Allow the paper to dry completely.
Observations
The chromatogram displays four distinct bands of colour, indicating the separation of the leaf pigments.
Result
The pigments within the mixture have separated into four distinct bands as follows, from the bottom up to the solvent front:
Band of green colour- chlorophyll b
Band of blue-green colour- chlorophyll a
Band of yellow colour- xanthophyll
Band of orange colour- carotene
Separation of leaf pigments using paper chromatography
Precautions
Do not use a ball or ink pen to mark the chromatography paper.
Ensure that the loaded spot is not touching the solvent and is visible above it
Make sure to transfer a generous amount of the sample onto the paper to ensure better results
Be careful not to handle ether acetone near any flame source, as this solvent is highly flammable.
Lab Manual Questions
1. Which photosynthetic pigment travels the farthest and why?
Ans: Carotene travels the farthest, near the solvent front. This is because it is more soluble in the solvent than the other three pigments and doesn’t bond to the medium of the paper via hydrogen bonds.
2. Why is acetone used as a solvent instead of water?
Ans: Acetone is less polar than water. This allows for greater allows greater resolution, i.e., better separation of the pigments in the mixture
3. What will happen if the spot touches the solvent?
Ans: It is important to ensure that the sample doesn’t touch the mobile phase. If it does, the mixture spot-loaded will dissolve immediately in the solvent, thereby maligning the entire experiment
Viva Questions
1. How many pigments have you separated from the sample? What are they?
Ans: Four pigments have been separated from the sample, chlorophyll a, chlorophyll b, xanthophyll and carotene
2. Why do you think acetone is used as the mobile phase in this experiment, not water?
Ans: Ether-acetone is a non-polar solvent. The natural pigments present in the leaves are, however, polar. This is important since separation in paper chromatography is based on the differing solubilities of the pigments in the solvent and the stationary phase.
3. Based on your experiment, what can you infer about the polarities of the separated pigments?
Ans: The most polar pigment is chlorophyll b, followed by chlorophyll a, xanthophyll, and finally, carotene, which is the least polar, hence most soluble in the non-polar solvent.
4. What do you know about the polarity of the chromatography paper?
Ans: The chromatography paper is made up of cellulose, attracting water vapour molecules, thereby contributing to the polar nature of the paper.
5. How many different types of pigments occur in plants? Can you name these pigments?
Ans: Four types of pigments occur in plants- the chlorophylls, and the carotenoids, which include carotene and xanthophylls like lutein, anthocyanin, and betalains.
6. We are well aware that chlorophylls are involved in photosynthesis. What are the functions of carotenoids?
Ans: Carotenoids are also involved in photosynthesis. They absorb wavelengths not received by chlorophylls and transfer the absorbed energy to the chlorophyll molecules. Hence, they are also called accessory pigments. They are also involved in photoprotection.
7. Apart from paper chromatography, what other types of chromatographies are used for separation purposes?
Ans: Thin layer chromatography, column chromatography, gas chromatography, ion-exchange chromatography, affinity chromatography, etc., are the other types used to separate components in a mixture.
8. What are the absorption spectrum values of chlorophyll a and b?
Ans: Chlorophyll a absorbs the maximum wavelength of 642 nm and 372nm (red and blue regions, respectively), while chlorophyll b absorbs a maximum wavelength of 626 nm and 372 nm (red and blue regions, respectively).
Practical Based Questions
The pigment that appears blue-green on the chromatogram is?
Chlorophyll a
Chlorophyll b
Xanthophyll
Carotene
Ans. A. Chlorophyll a
The pigment that is most soluble in the solvent is:
Chlorophyll a
Chlorophyll b
Xanthophyll
Carotene
Ans. D. Carotene
The stationary phase employed in the paper chromatography experiment is:
Acetone
Ether-acetone
Water
Paper
Ans. D. Paper
Which of the following is not involved in separating leaf pigments in paper chromatography?
Adsorption
Solubility
Capillarity
Gravitation
Ans. D. Gravitation
Xanthophyll belongs to _____ group of plant pigments
Chlorophylls
Porphyrins
Carotenoids
Flavonoids
Ans. C. Carotenoids
The P700 reaction centre comprises
More of chlorophyll a than chlorophyll b
More of chlorophyll b than chlorophyll a
Equal amounts of chlorophyll a
Only chlorophyll a
Ans. A. More of chlorophyll a than chlorophyll b
The pigment that is least soluble in the mobile phase is
Chlorophyll a
Chlorophyll b
Carotene
Xanthophyll
Ans. B. Chlorophyll b
The pigment that travels farthest on the paper is
Most soluble in the solvent
Least soluble in the solvent
Not soluble in the solvent
Of lower molecular weight than the other pigments
Ans. A. Most soluble in the solvent
Conclusion
A leaf from a typical photosynthetic plant comprises four pigments: chlorophyll a and b, xanthophyll and carotene.
These pigments can be separated using the paper chromatography technique, using acetone as the mobile phase/ solvent.
Four bands of separated pigments are observed, with chlorophyll b at the bottom, followed by chlorophyll a, xanthophyll and finally, carotene at the top.
FAQs on Class 11 Biology Separation Of Plant Pigments Through Paper Chromatography Experiment
1. What are the most important points to remember when answering questions on the separation of plant pigments through paper chromatography in the CBSE Class 11 Biology exam?
When answering such questions for board exams, focus on:
- The principle of paper chromatography (partition between stationary and mobile phases)
- Names, colours, and relative positions of pigments separated (chlorophyll a, chlorophyll b, xanthophyll, carotene)
- The reason acetone or ether-acetone is used as the mobile phase
- Definition and calculation of the Rf value
- Key precautions during the experiment (spot application, safety, solvent handling)
2. Which type of exam questions on plant pigment chromatography commonly carry higher marks and how should students approach them?
Questions that carry 5 marks in Biology often ask for the detailed procedure, explanation of principle, diagram of chromatogram, and results observed. To score well, structure answers with clear steps, diagrams, and precise science-based explanations. Use labelled diagrams and mention at least four separated pigments, their colours, and order on the chromatogram.
3. Why is acetone preferred over water in the separation of plant pigments during paper chromatography for board exam questions?
Acetone is preferred because it has lower polarity than water, allowing for better separation of both polar and non-polar pigments present in leaves. This leads to sharper, more distinct bands, which is essential in demonstrating clear chromatographic results as expected in board answers.
4. What marks scoring traps should students avoid while writing about the chromatography of leaf pigments in exams?
Common traps include:
- Not defining the principle of chromatography clearly
- Omitting or mislabelling pigments on the diagram or in the explanation
- Missing safety precautions, especially regarding the use of flammable solvents
- Failing to mention proper sample application (not touching the solvent line)
- Skipping the definition or significance of Rf value
5. What is the exam-relevant significance of calculating the Rf value in the separation of plant pigments through paper chromatography?
The Rf value (Retention factor) is important for distinguishing and identifying different pigments based on how far each travels relative to the solvent front. In exams, expressing Rf as a formula and giving sample values demonstrates both understanding and application of concepts, which is often evaluated in 3-mark and 5-mark questions.
6. How can students effectively illustrate and label a chromatogram of plant pigments for full marks?
To earn full marks:
- Draw the filter paper strip and clearly indicate the origin line, solvent front, and four pigment bands
- Label each band with pigment names and colours: Chlorophyll b (green), Chlorophyll a (blue-green), Xanthophyll (yellow), Carotene (orange)
- Add the direction of solvent movement and mention the mobile and stationary phases
7. What are the most expected higher order thinking questions (HOTS) related to plant pigment chromatography in recent board exams?
Common HOTS include:
- Explaining what would happen if a more polar solvent was used instead of acetone
- Predicting the effect of different plant samples on the chromatogram pattern
- Analyzing why some pigments travel farther than others based on solubility and polarity
- Comparing chromatographic separation with other methods like thin layer chromatography
8. Why does carotene appear at the top of the chromatogram in the separation of plant pigments, and how can this knowledge be used in exam answers?
Carotene appears at the top because it is the most soluble in the non-polar solvent and least attracted to the polar cellulose paper. Stating this demonstrates an understanding of separation based on solubility and polarity, an aspect frequently tested in board questions.
9. In the context of board marking schemes for this practical, how important are precautions and observational details in answers?
Precautions and detailed observations are crucial. Examiners allocate marks for correctly stating procedural safety (e.g., avoiding fire when handling acetone), sample spot handling, and specific band positions. Including 2-3 key precautions can help secure full marks for these sections in 3- or 5-mark questions.
10. How should students address a 3-mark question comparing paper chromatography of leaf pigments to other chromatographic techniques in biology exams?
Structure the answer as:
- Paper chromatography separates based on partition between stationary and mobile phases
- Other methods like thin layer chromatography (TLC) and column chromatography use different stationary phases and can separate a wider variety of compounds
- Pigment separation on paper is visual and rapid, but less suitable for quantitative analysis compared to modern methods
























