




An Overview of Class 11 Chemistry Viva Questions With Answers On Determination Of Boiling Point Experiment
The boiling point (b.p.) of a liquid is defined or described as the temperature at which the pressure or vapour pressure of the same liquid is equal to the atmospheric pressure exerted or applied on the surface of the liquid. Pressure cookers are the most common example of boiling in our everyday life. Also, boiling water makes it pure and filtered without filtration methods like charcoal treatment, filter paper, etc.
Table of Content
Aim
Materials Required
Theory
Procedure
Observations
Result
Precautions
Aim
To determine the boiling point of the given substances and also create viva questions and answers for this.
Materials Required
Thermometers, (Celsius scale)
Boiling tube
A glass rod
Iron stand
A Bunsen burner
Wire gauze
Beakers
Tripod Stand
Distilled water
Theory
1. Boiling Point
The temperature at which the liquid boils and changes into a gaseous state at atmospheric pressure is called the boiling point. For example, water boils at 100 °C to form water vapour (at 76 cm pressure).
2. Latent Heat of Vapourization
The heat energy absorbed by the water when it changes its phase to steam, this hidden heat, is called the latent heat of vapourization.
Procedure
Take 25-30 ml of water in a boiling tube and add a few pumice stones to it.
Clamp the boiling tube on an iron stand with two holed corks, in one hole fix the thermometer and in the other one fix the delivery tube.
Place the thermometer above the water in the flask as shown in the figure and record its temperature.
Place a burner under the boiling tube.
Read the temperature and record it in the given observation table till the water boils. Record the reading after the time interval of 1 minute.
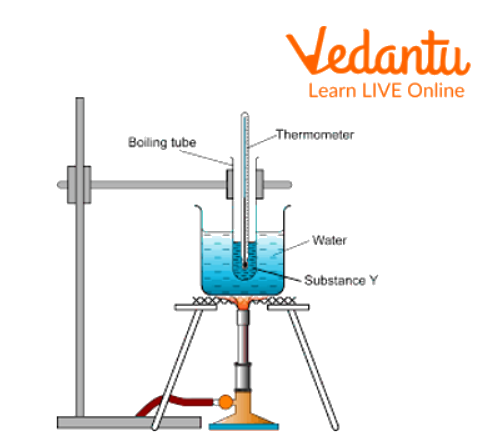
Set-up of the Apparatus
Observations
Result
The boiling point of water is 100 °C.
Once the boiling point is attained, the temperature reading on the thermometer does not change for some time.
Precautions
Choose a better quality thermometer whose graduated scale is readable.
Record the temperature in whole numbers.
While reading the thermometer, the eye level should be parallel with the mercury level.
Dip only the bulb of the thermometer into water/ice.
The thermometer should not touch the walls of the beaker or boiling tube.
Lab Manual Questions
1. For proper reading, what should be the position of the thermometer bulb and ignition tube?
Ans. For proper reading, the thermometer bulb and the ignition tube (lower end) should be placed at the same level.
2. The sharp boiling point of crystalline solids is due to the___________.
Ans. Crystalline solids have a sharp boiling point because all the components of the crystalline solid are present at the same or equal distance from each other.
3. Which metal block has been used in this experiment?
Ans. An aluminium block has been used in this experiment.
4. The boiling point of a pure substance is sharp because of the__________.
Ans. The boiling point of a pure substance is sharp because pure substances exhibit very well-defined physical properties or properties that are not connected with the substance’s ability to combine with the different substances.
Viva Questions
1. Define boiling point.
Ans. The boiling point may be defined as the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure.
2. On increasing pressure, how does the boiling point gets affected?
Ans. On increasing the outside pressure, the boiling point of the liquid increases.
3. What is the effect of a decrease in pressure on the boiling point?
Ans. On decreasing the outside pressure, the boiling point of the liquid decreases.
4. What will happen to the boiling point of the liquid if some non-volatile liquid is added to it?
The boiling point of the liquid will increase.
5. Why do different liquids have different boiling points?
The boiling point depends upon intermolecular forces existing in the liquid. Since different liquids have intermolecular forces of different strengths, therefore their boiling points are different.
6. Why is food cooked more quickly in a pressure cooker?
In a pressure cooker, water boils at a higher temperature and hence cooking takes place at a higher temperature.
7. Suppose the boiling point of a liquid is 100 °C in Kolkata. At the hill station will it be the same or different? Give reasons.
The boiling point of the liquid will be less than 100 °C at the hill station. The boiling point decreases with a decrease in atmospheric pressure. At hill stations, the atmospheric pressure is less than that in plains.
8. Why do carboxylic acids have a higher boiling point than hydrocarbons?
Ans. The carboxylic acid can form hydrogen bonding, and these hydrogen bonds stabilize the complete molecule, enabling the organic chain to form additional bonds by dispersion forces.
9. Define the effect of impurity on boiling point.
Ans. Impurities increase the boiling point. This is due to the reason that it stabilizes the liquid phase and makes it more energetically favourable.
10. At what temperature is the density of water maximum?
Ans. At 4 °C, the density of water is maximum.
Practical Questions
1. The boiling point of pure water is-
0 ℃
100 F
100 ℃
None of these
Ans. The boiling point of water or pure water is 100 ℃.
2. What is happening at the boiling point
Liquid to gas
Solid to liquid
Gas to liquid
Liquid to solid
Ans. At boiling point, liquid changes or converts into gas.
3. The boiling point of water in hilly regions?
Less than 100 ℃
Equal to 100 ℃
Greater than 100 ℃
None of these
Ans. In a hilly region, the boiling point of water is less than 100 ℃.
4. How does the boiling point relate to atmospheric pressure?
Directly proportional
Indirectly proportional
Not related
None
Ans. The boiling point and atmospheric pressure are directly proportional to each other.
5. The boiling point of water at sea level is-
99 ℃
100 ℃
103.7 ℃
273 ℃
Ans. At sea level, the boiling point of water is 100 ℃.
6. How does the boiling point get affected by the molecular weight?
It decreases with an increase in molecular weight.
It increases with an increase in molecular weight.
It increases with a decrease in molecular weight.
Does not get affected
Ans. The boiling point increases with an increase in molecular weight.
7. Which of the following possesses the highest boiling point?
Butane
Methane
Propane
Pentane
Ans. Pentane has the highest boiling point of all.
8. How is the boiling point impacted by the hydrogen bonding?
Decreases with an increase in hydrogen bonding.
Increases with an increase in hydrogen bonding.
Increases with a decrease in hydrogen bonding.
Having no effect on hydrogen bonding.
Ans. The boiling point increases with an increase in hydrogen bonding.
9. Camphor is used in molecular mass determination because
It has a very high cryoscopic constant
It is volatile
It is readily available
It is a solvent for organic substances
Ans. Camphor is used in molecular mass determination because it is volatile.
Conclusion
From the above experiment, we learned to determine the boiling point of water. We can conclude that the boiling point of water is 100 °C. Once the boiling point is attained, the temperature reading on the thermometer does not change for some time.
FAQs on Class 11 Chemistry Viva Questions With Answers On Determination Of Boiling Point Experiment
1. What is the definition of boiling point, and how do intermolecular forces affect it for a 2-mark question in the Class 11 exam?
The boiling point of a liquid is the specific temperature at which its vapour pressure equals the external atmospheric pressure. For exams, remember that the strength of intermolecular forces is directly proportional to the boiling point. Liquids with stronger forces (like hydrogen bonds in water) require more energy to break these bonds and boil, resulting in a higher boiling point compared to liquids with weaker forces (like van der Waals forces in methane).
2. For the CBSE Class 11 Chemistry practical exam 2025-26, what is the expected effect on the boiling point of a liquid when a non-volatile solute is added?
Adding a non-volatile solute (like salt or sugar) to a pure solvent (like water) will increase its boiling point. This phenomenon is known as the elevation of boiling point. The solute particles occupy space at the liquid's surface, lowering its vapour pressure. Therefore, a higher temperature is required to make the vapour pressure equal to the atmospheric pressure.
3. Explain the relationship between boiling point and external atmospheric pressure. Why would a liquid boil at a lower temperature in Shimla than in Mumbai?
The boiling point of a liquid is directly dependent on the external atmospheric pressure. A liquid boils when its vapour pressure equals this external pressure.
- At high altitudes like Shimla, the atmospheric pressure is lower. Consequently, the liquid needs less heat to raise its vapour pressure to match the surrounding pressure, causing it to boil at a lower temperature.
- In coastal areas like Mumbai, the atmospheric pressure is higher, so more heat is needed, and the boiling point is higher.
4. What are two essential precautions a student must take while determining the boiling point of an organic liquid in the lab?
For practical exams, two critical precautions are:
- The bulb of the thermometer should be positioned so that its upper end is just below the side arm of the distillation flask. This ensures it measures the temperature of the vapour that is in thermal equilibrium with the boiling liquid, not the liquid itself.
- Add small pieces of porcelain or boiling chips to the liquid before heating. This ensures smooth, even boiling and prevents bumping or superheating of the liquid.
5. How is the correct boiling point identified during the experimental determination using a Thiele's tube?
The correct boiling point is the temperature at which a continuous and rapid stream of bubbles starts emerging from the lower end of the sealed capillary tube dipped in the liquid. The reading on the thermometer should be taken at this exact moment when the vapour pressure of the liquid becomes equal to the atmospheric pressure.
6. Why is a liquid heated in a paraffin or oil bath (like a Thiele's tube) instead of being heated directly on a flame for boiling point determination?
Heating the liquid in a bath (like a Thiele's tube filled with paraffin or sulphuric acid) ensures uniform and controlled heating. Direct heating can cause localised overheating and bumping, leading to inaccurate temperature readings. An oil bath distributes the heat evenly around the test tube, providing a more precise measurement of the true boiling point, which is a critical aspect of the experiment.
7. Is it possible for water to boil at a temperature other than 100°C? Explain the conditions.
Yes, it is a common misconception that water always boils at 100°C. This value is only true at standard atmospheric pressure (1 atm). The boiling point depends entirely on the external pressure.
- In a pressure cooker, the pressure is increased, so water boils at a temperature above 100°C.
- At high altitudes or in a vacuum chamber where pressure is reduced, water can boil at temperatures well below 100°C.
8. What is 'superheating' and how does it lead to an error in determining the boiling point?
Superheating is a state where a liquid is heated to a temperature above its normal boiling point without actually boiling. This happens when there are no nucleation sites for bubbles to form. It can cause a sudden, violent boiling (bumping) when a bubble finally forms, leading to a sudden temperature drop. This results in an inaccurate and erroneously high boiling point reading. This is why boiling chips are added to provide surfaces for smooth bubble formation.
























