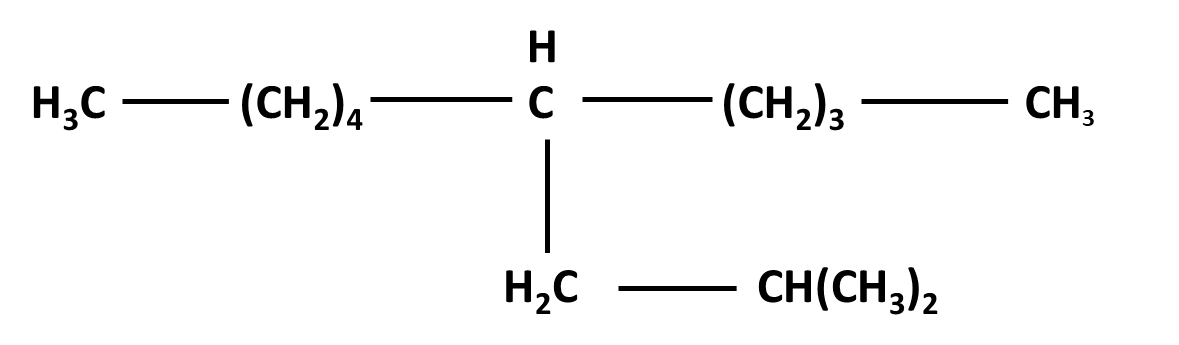
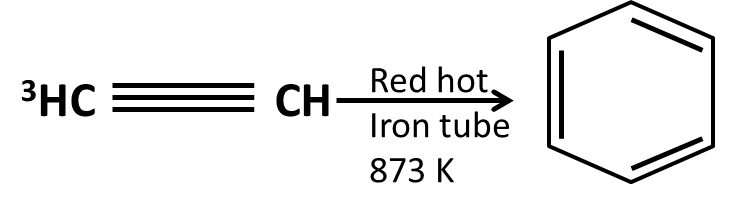
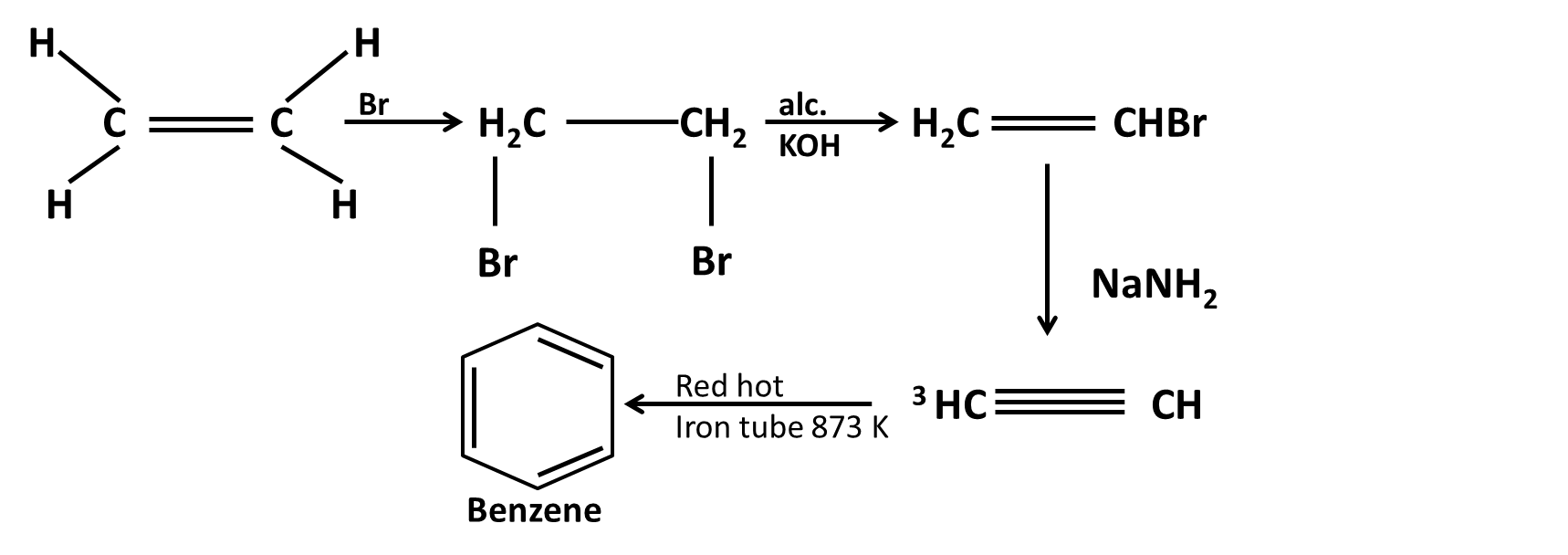
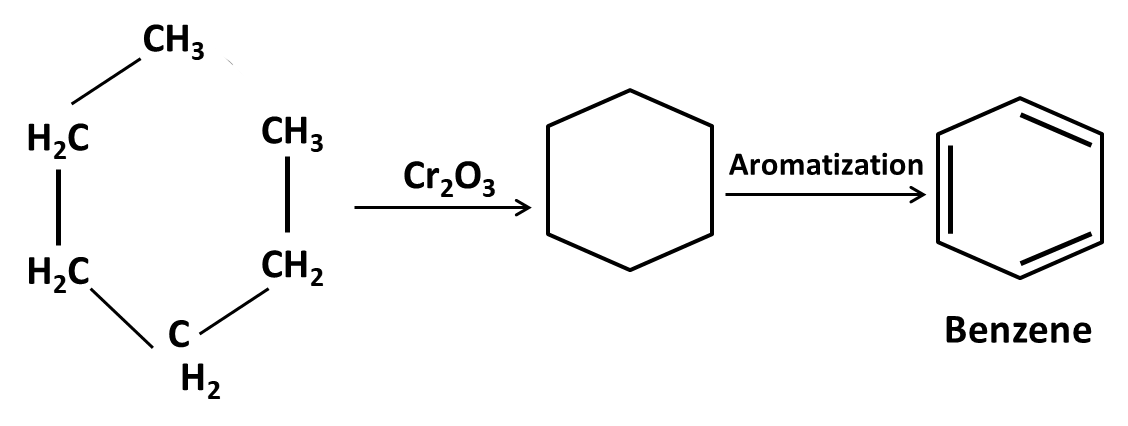
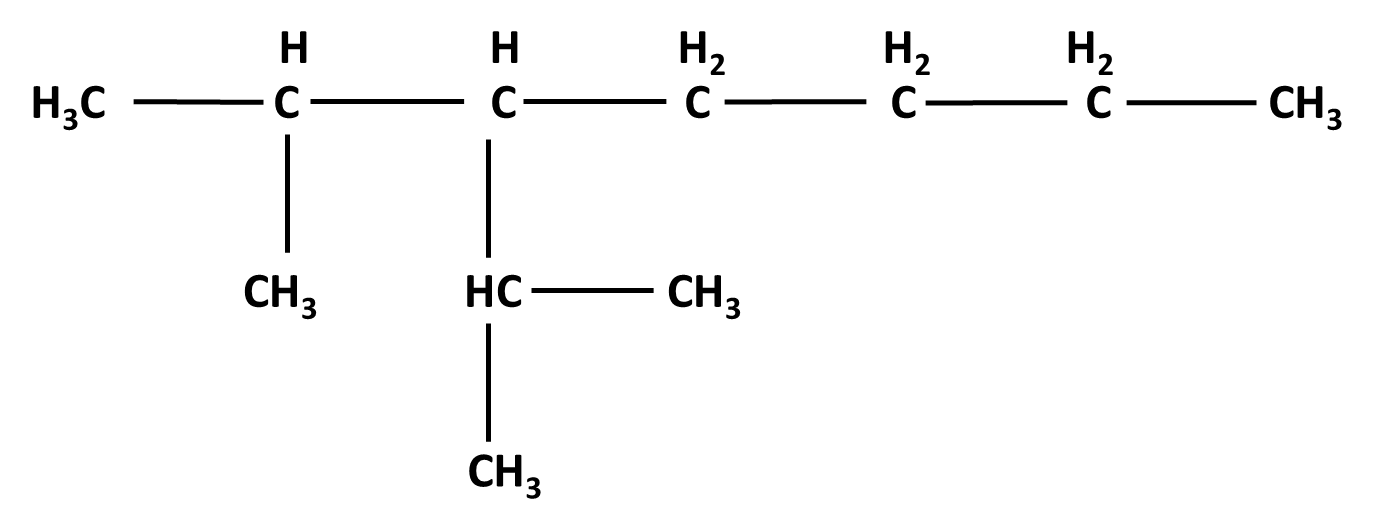
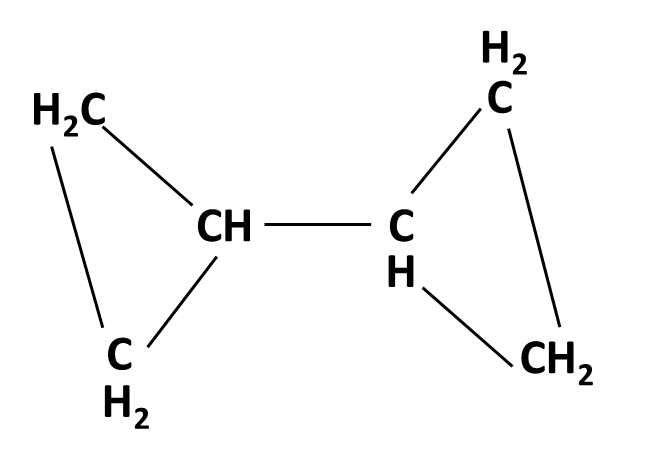
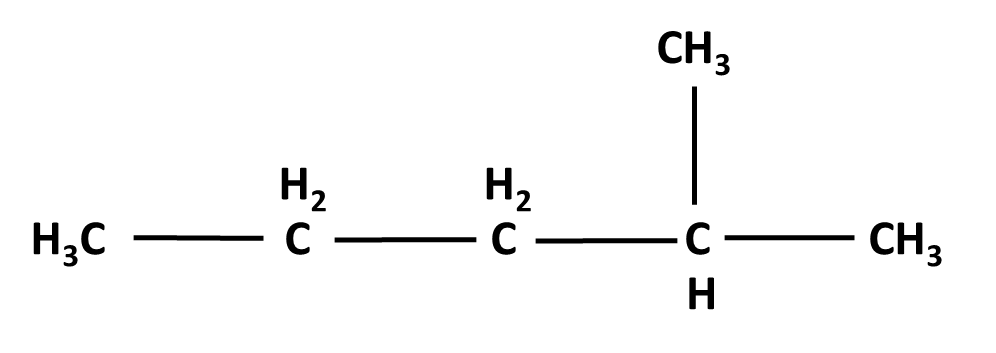
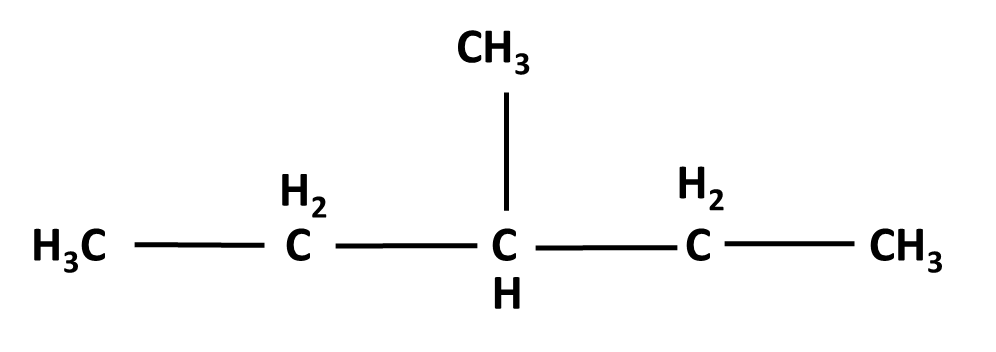
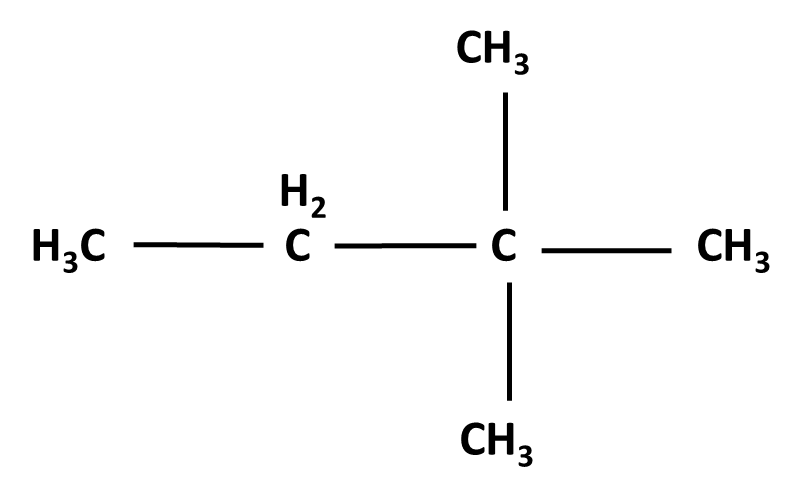
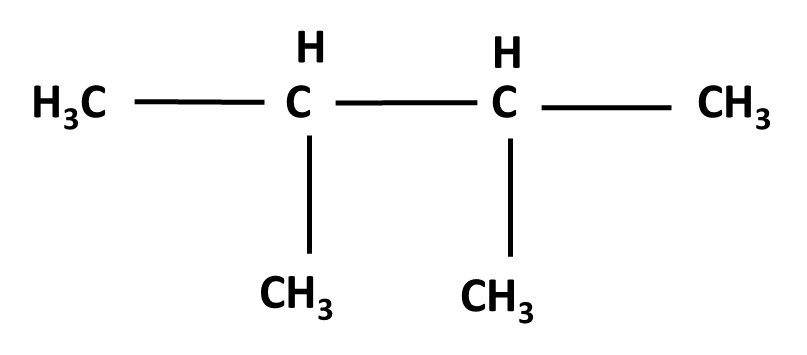
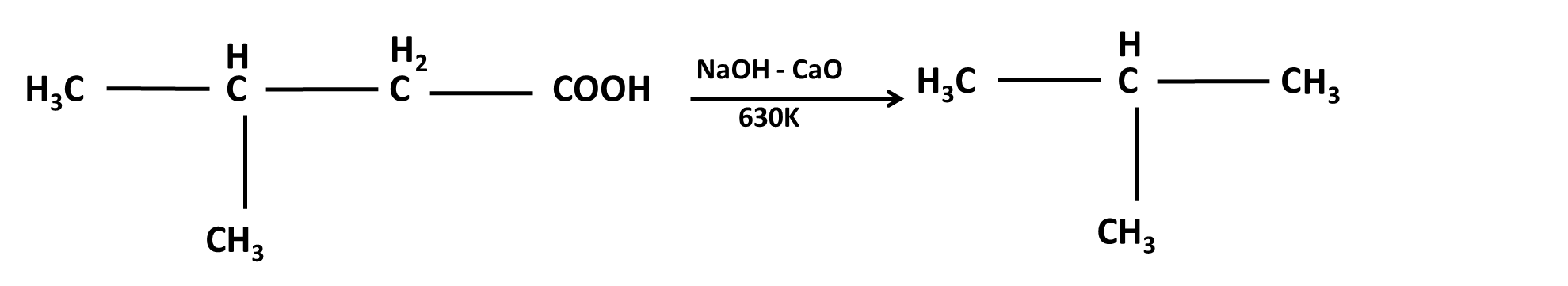
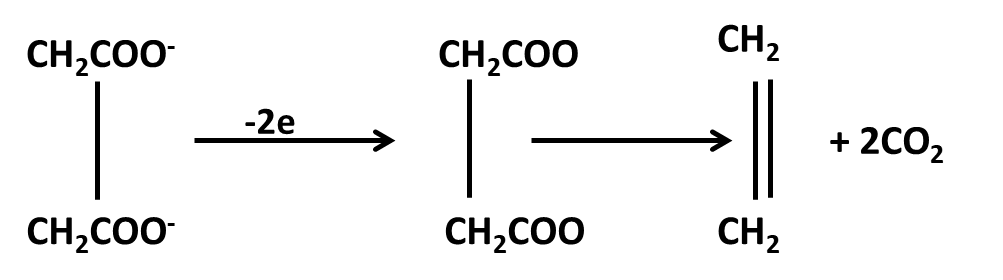
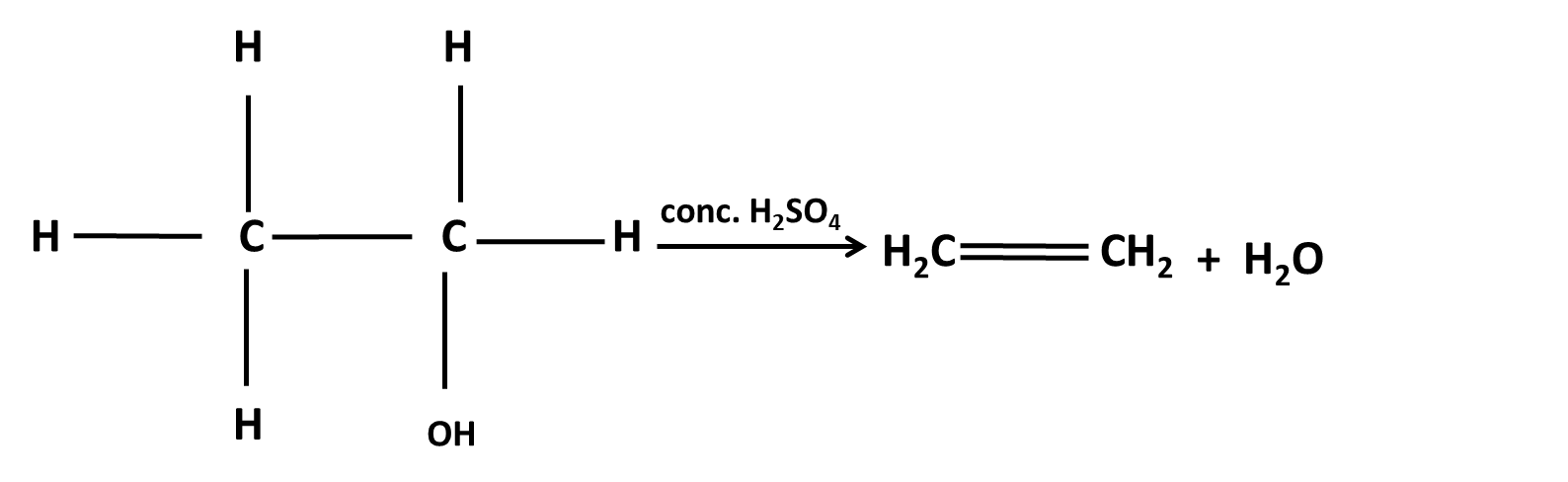
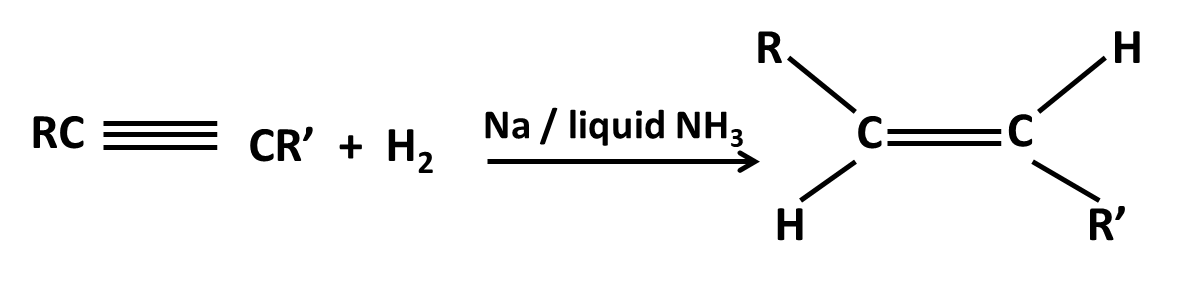
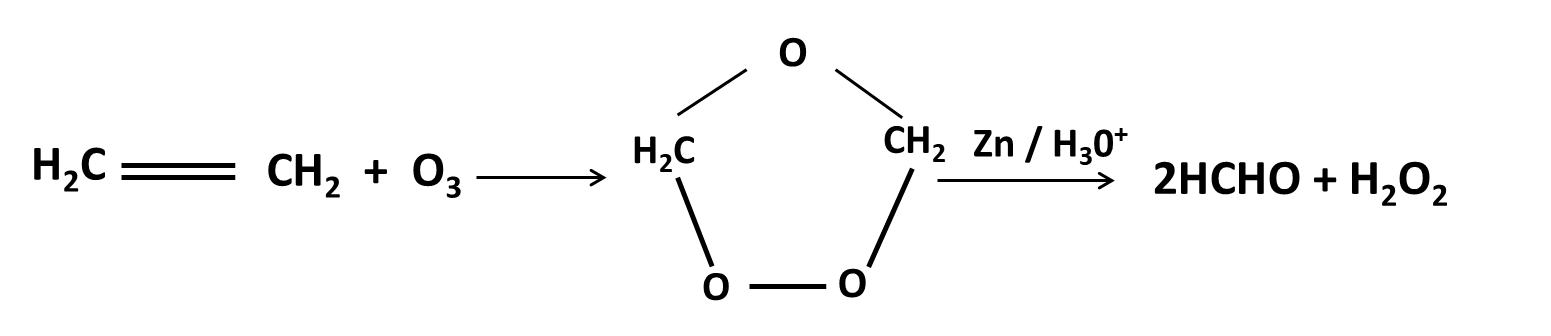
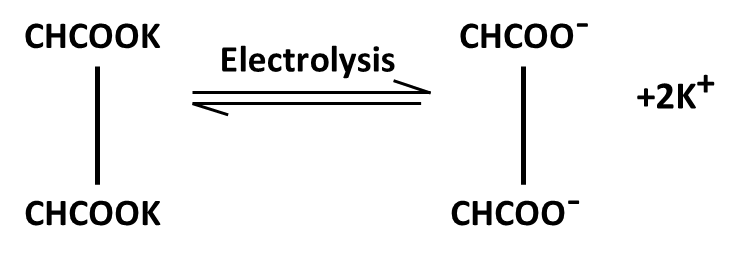
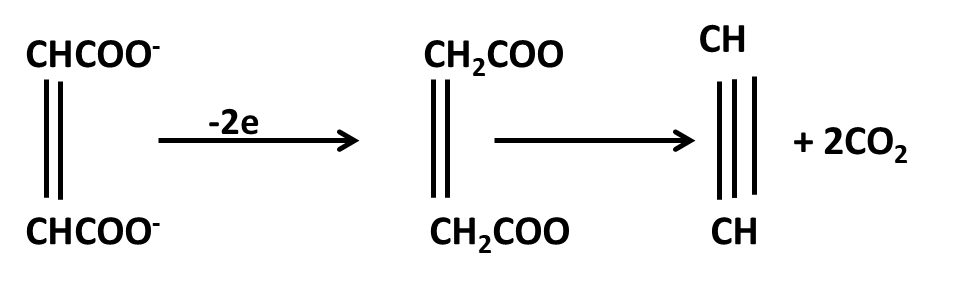
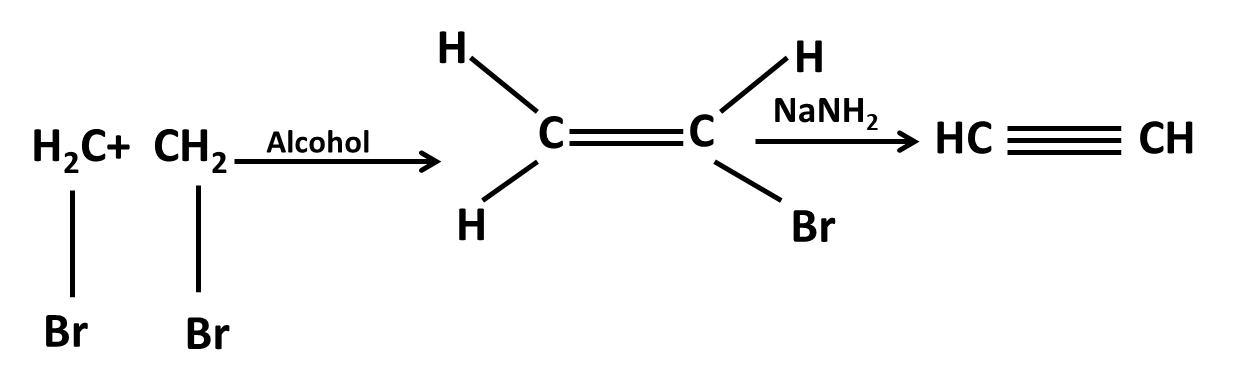
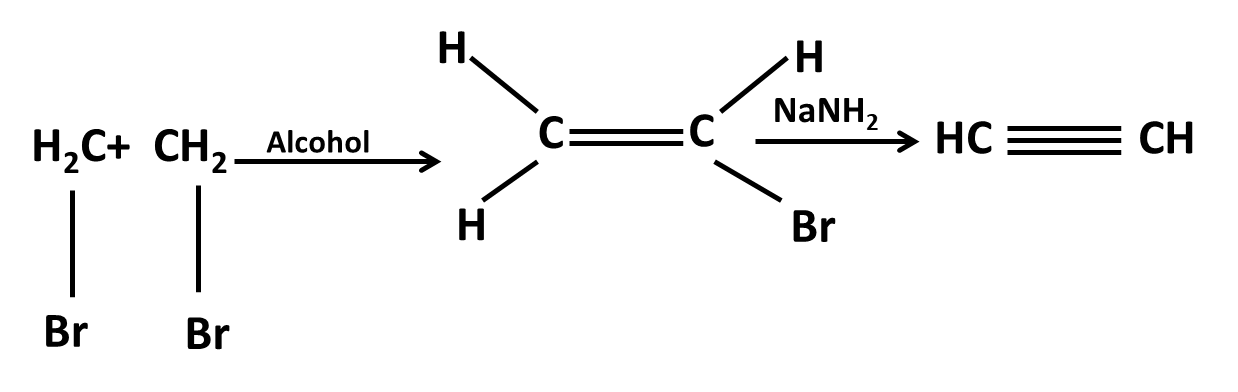
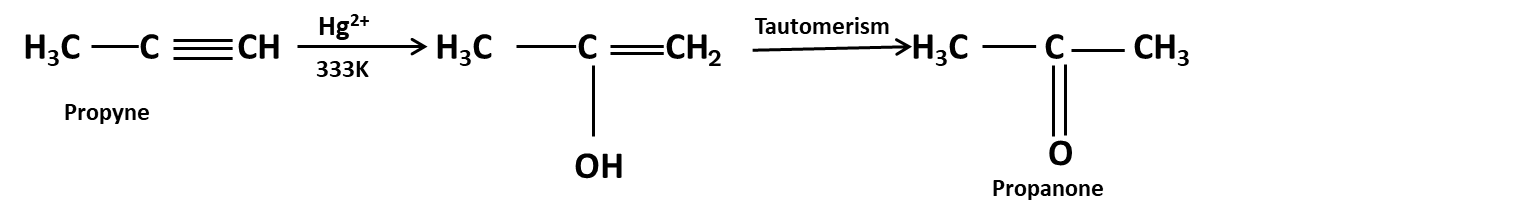
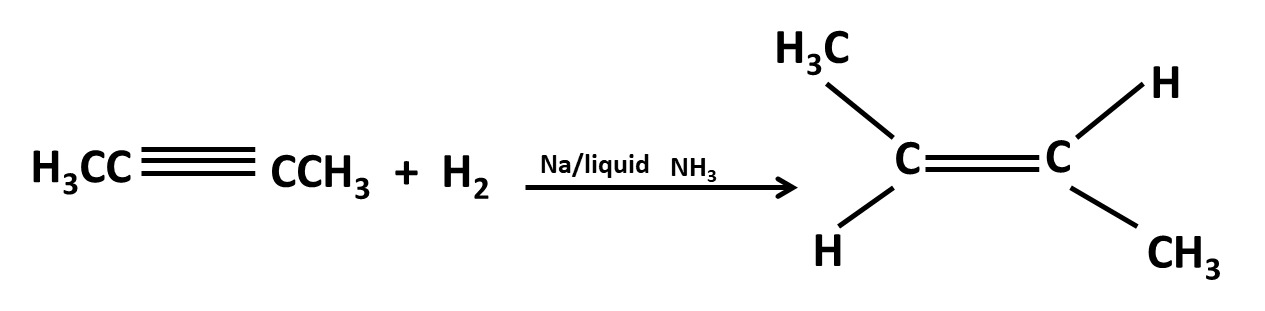
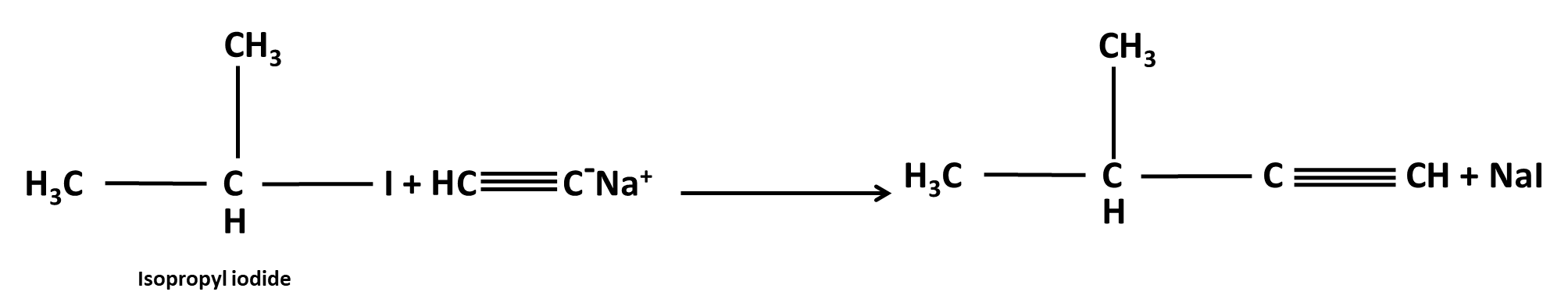
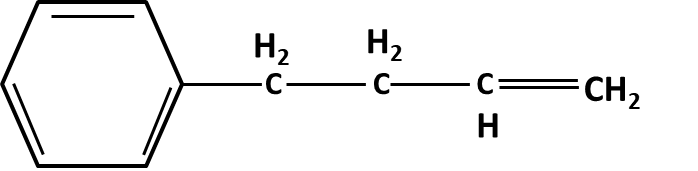
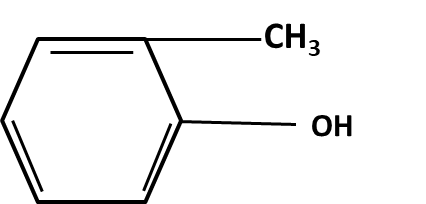
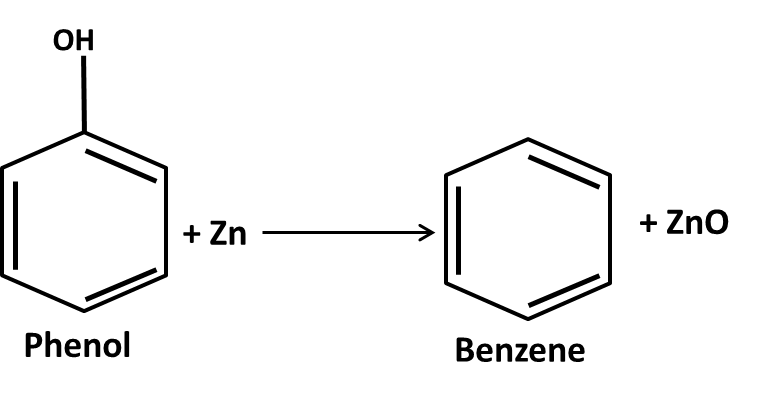
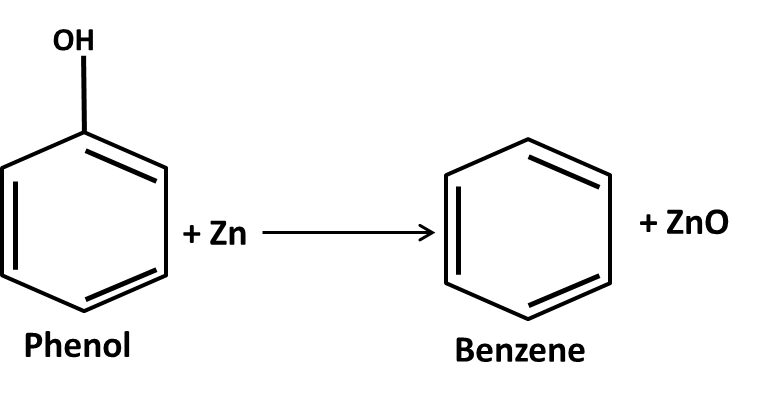
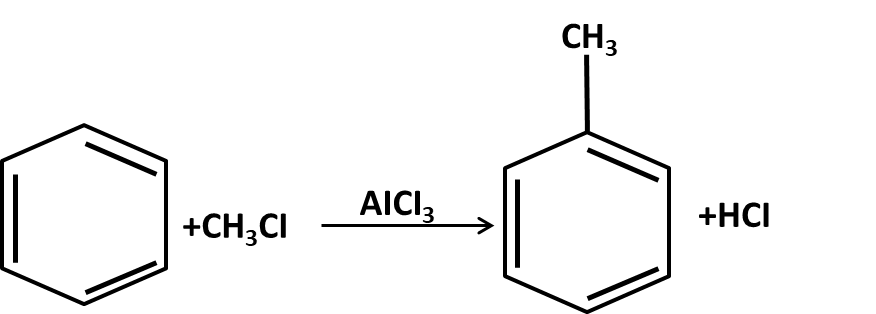
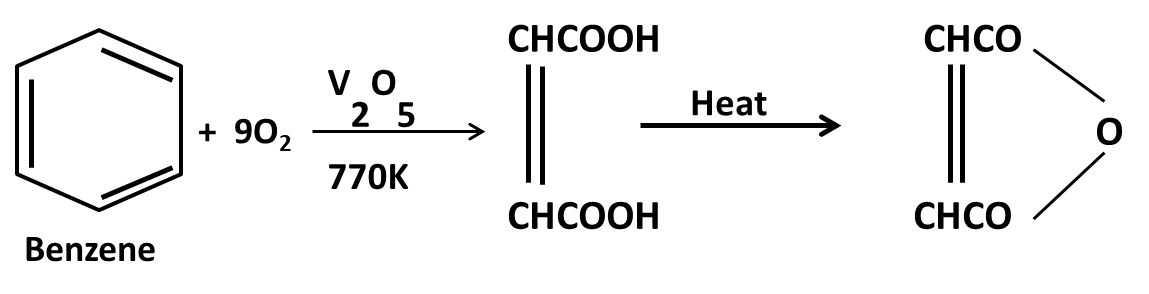
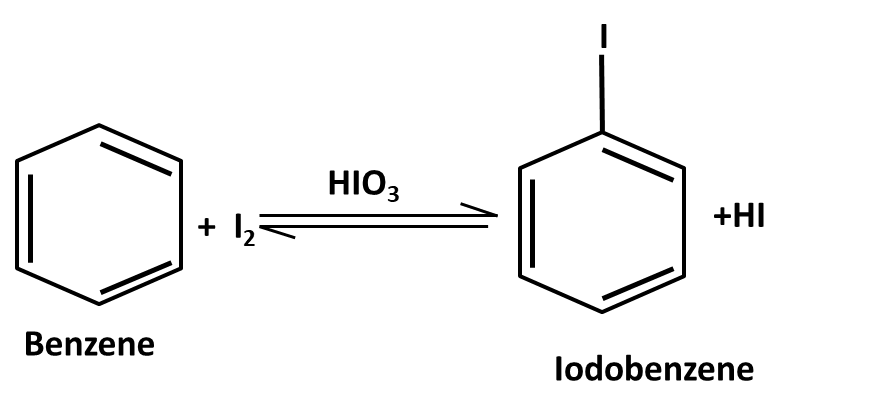
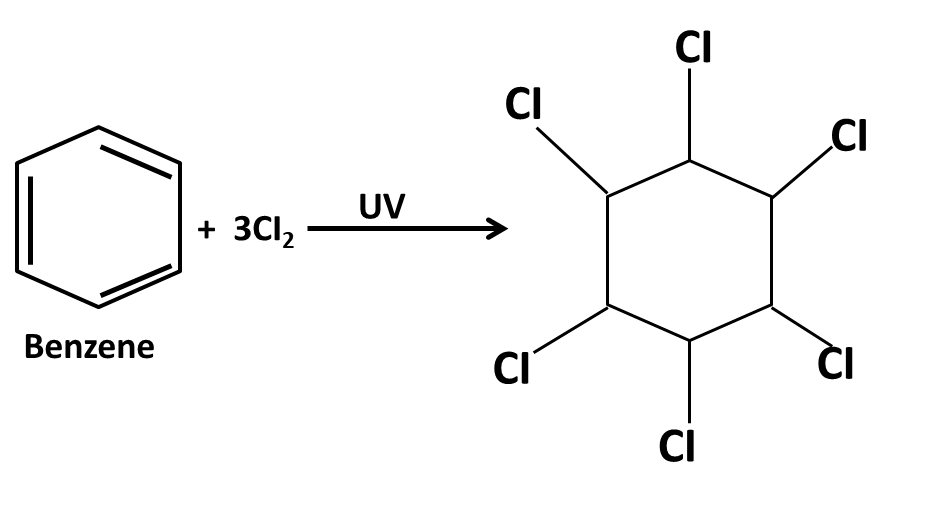
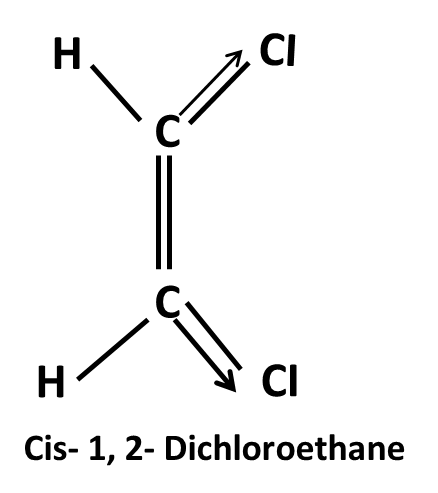
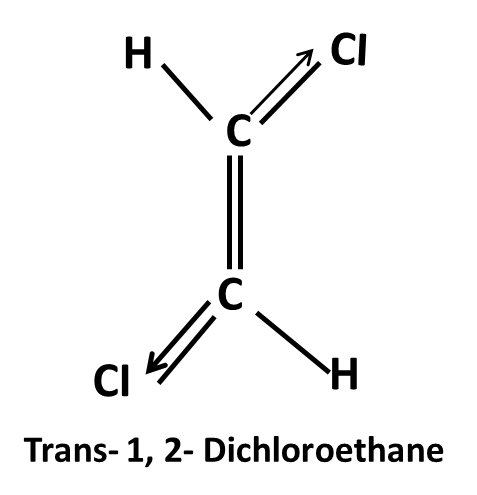
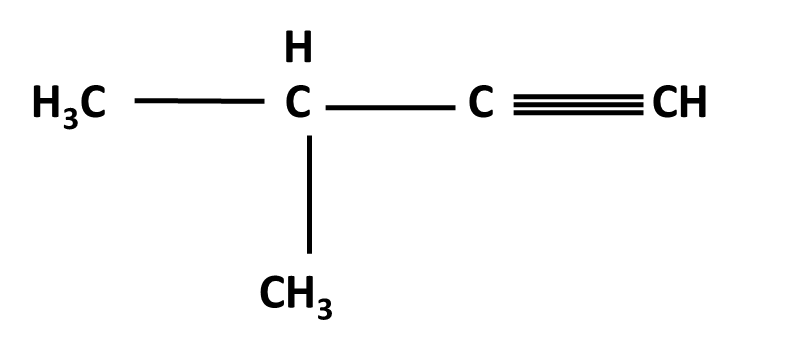
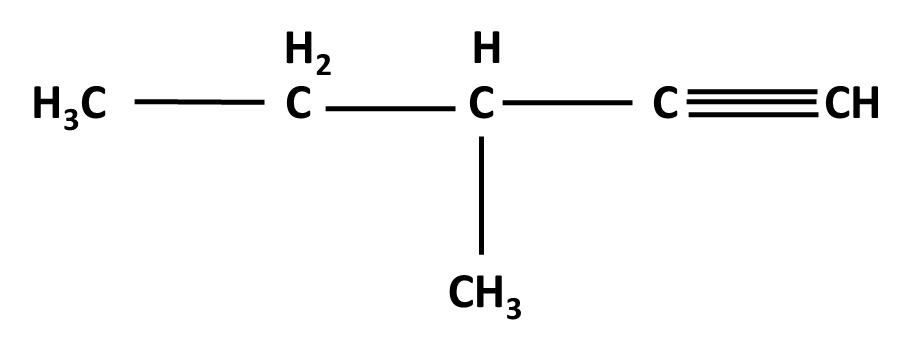
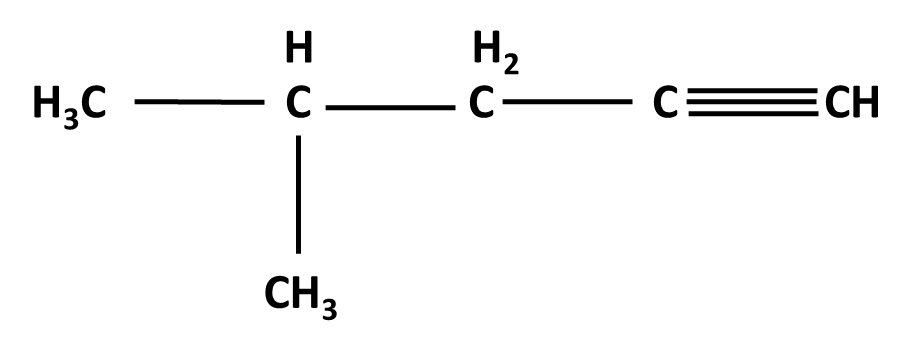
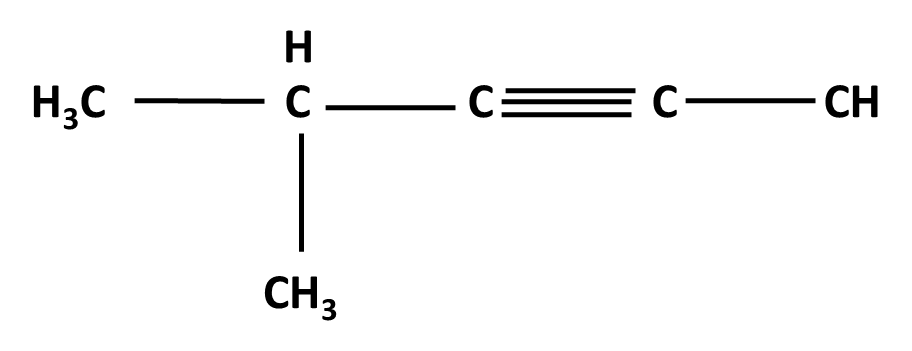
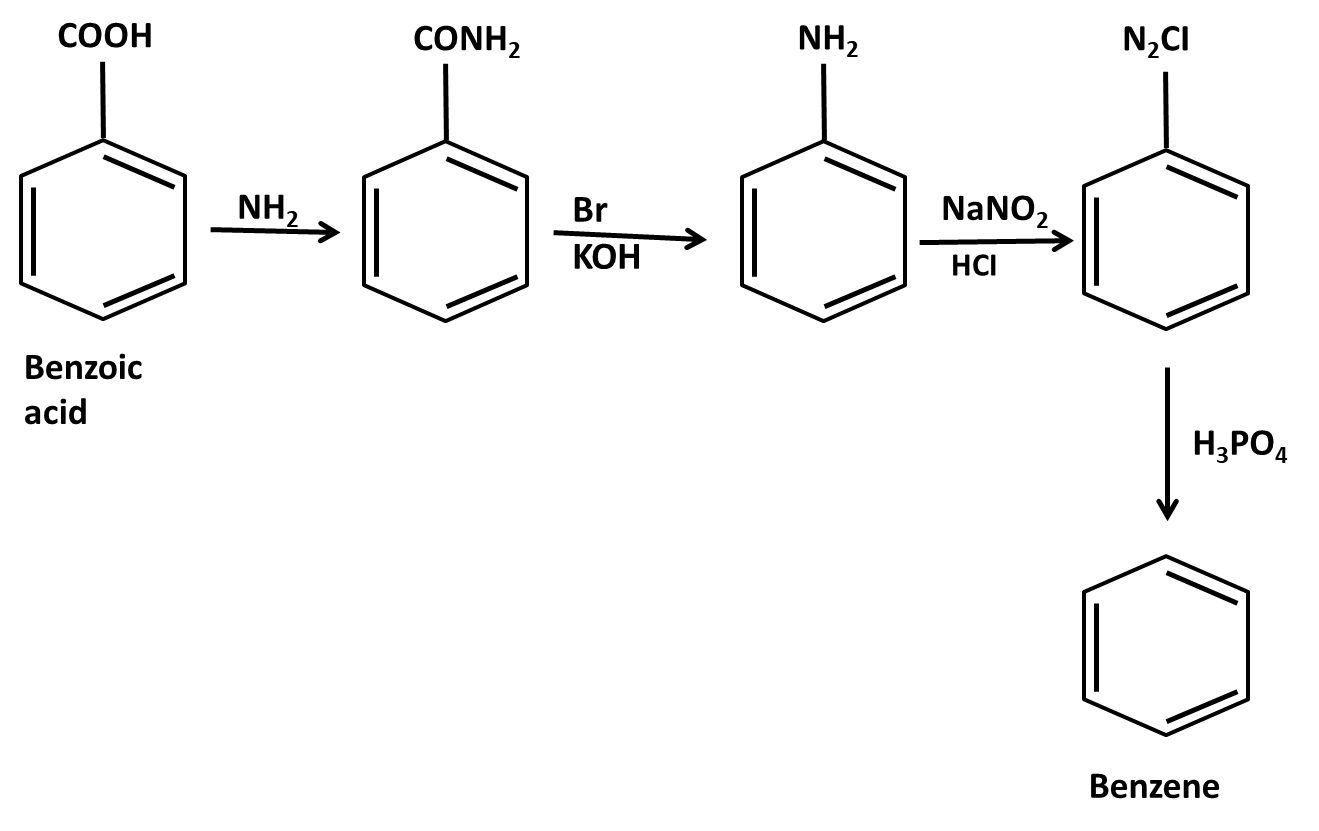
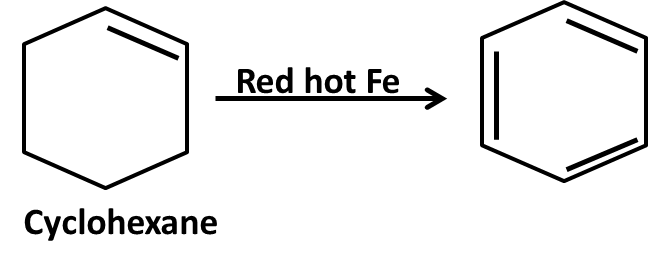
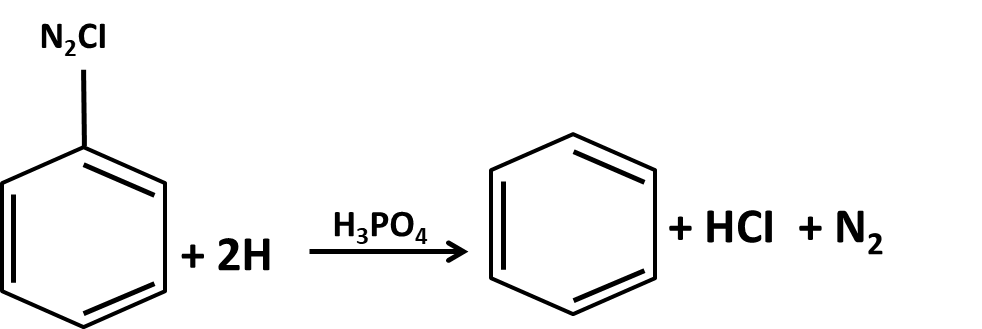
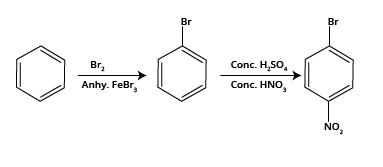
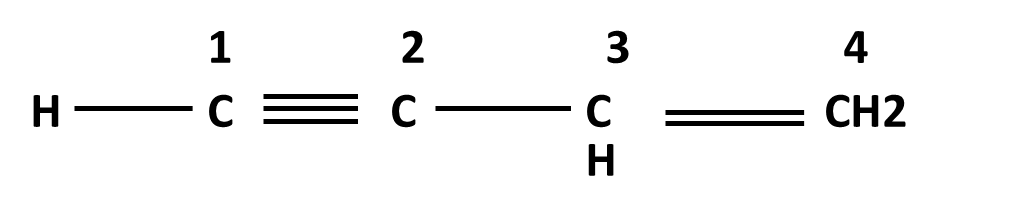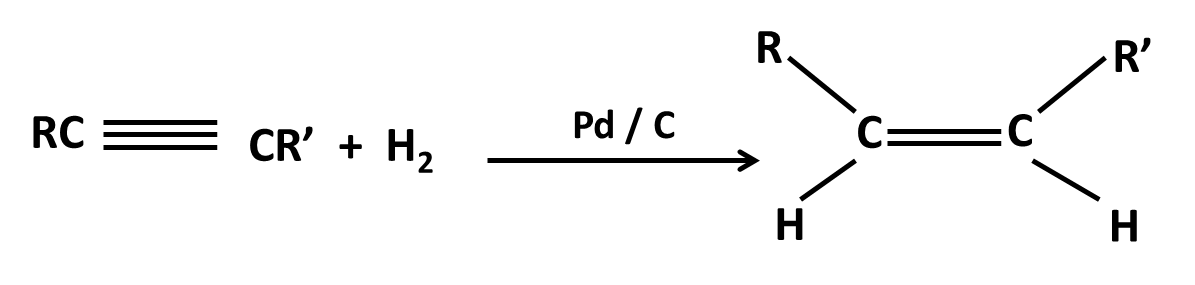Hydrocarbons Class 11 important questions with answers PDF download
FAQs on CBSE Important Questions for Class 11 Chemistry Hydrocarbons - 2025-26
1. What are the most important topics to focus on for Hydrocarbons Class 11 important questions as per CBSE 2025–26?
The key areas for Hydrocarbons Class 11 important questions include:
- Classification of hydrocarbons (alkanes, alkenes, alkynes, aromatic)
- IUPAC nomenclature and isomerism in hydrocarbons
- Preparation and properties of alkanes, alkenes, alkynes
- Reactions: Substitution, addition, elimination, and unique aromatic reactions
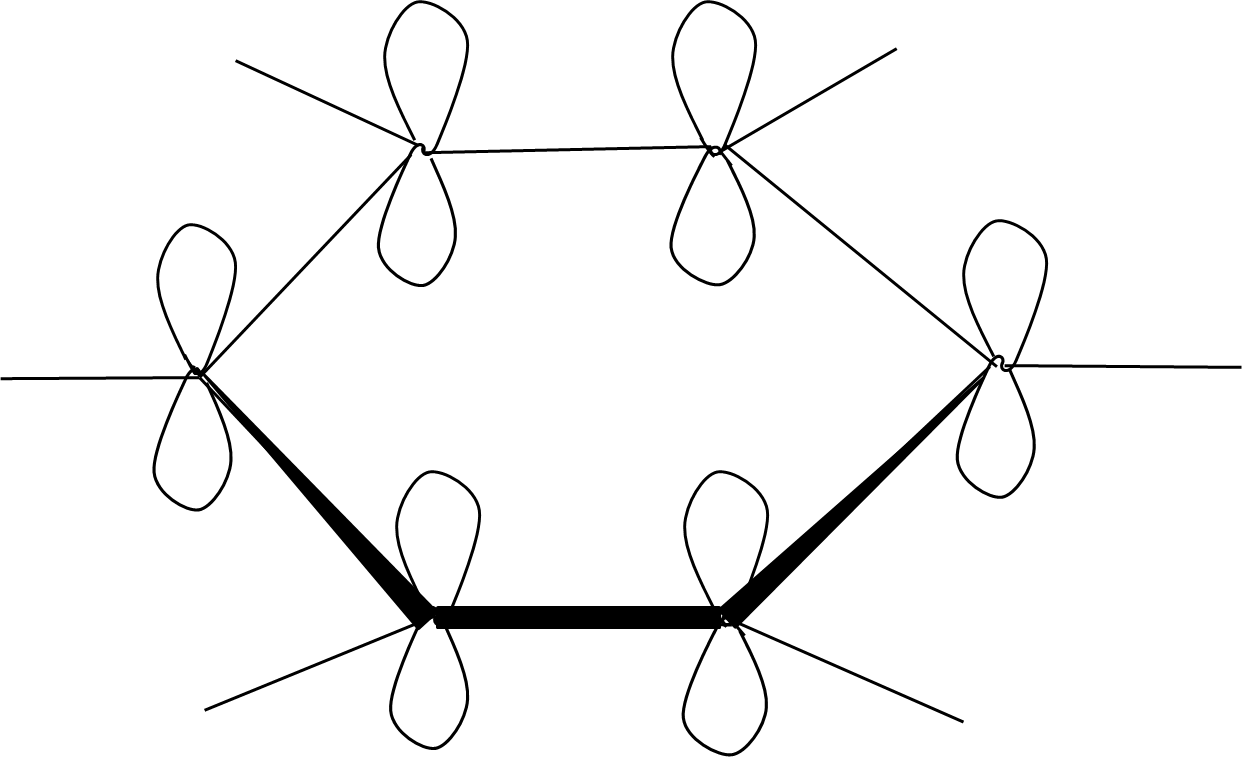
- Concepts of resonance and aromaticity (Huckel’s Rule, benzene structure, delocalization)
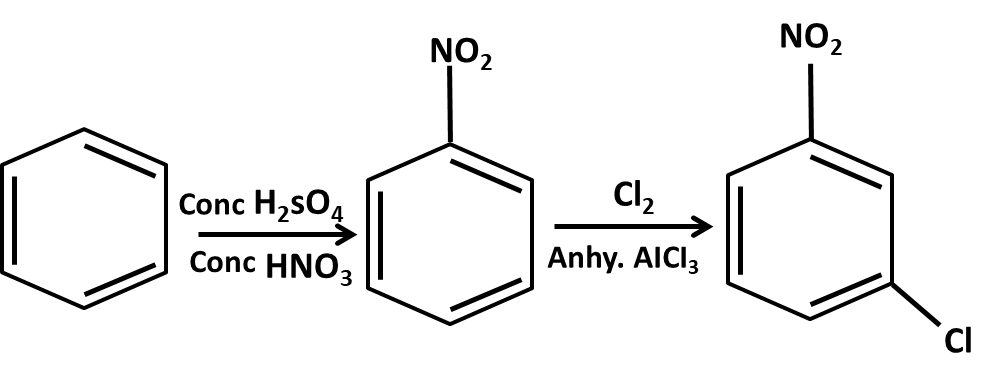
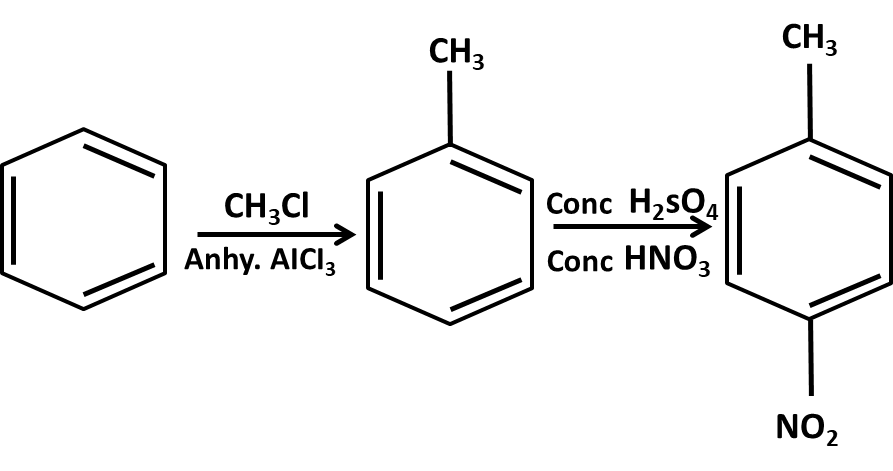
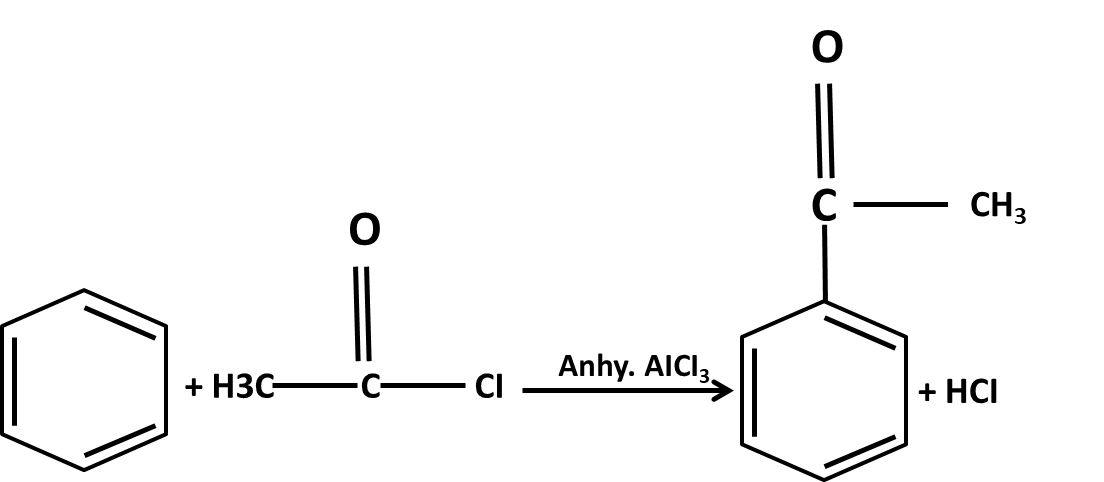
- Mechanisms of key name reactions (Markovnikov, anti-Markovnikov, Wurtz, Friedel–Crafts)
- Practical applications and difference between aliphatic and aromatic hydrocarbons
2. Which types of questions are most frequently asked from Hydrocarbons in CBSE Class 11 exams?
- IUPAC naming and structure drawing (1–2 mark questions)
- Mechanisms (stepwise process, especially addition and substitution reactions)
- Conversions and interconversions (e.g., converting one hydrocarbon type to another)
- Distinguishing tests for different hydrocarbons (like Baeyer's reagent, bromine water)
- Explaining resonance, aromaticity, and exceptional stability
- Reasoning-based HOTS (Higher Order Thinking Skills) regarding physical properties and reactivity trends
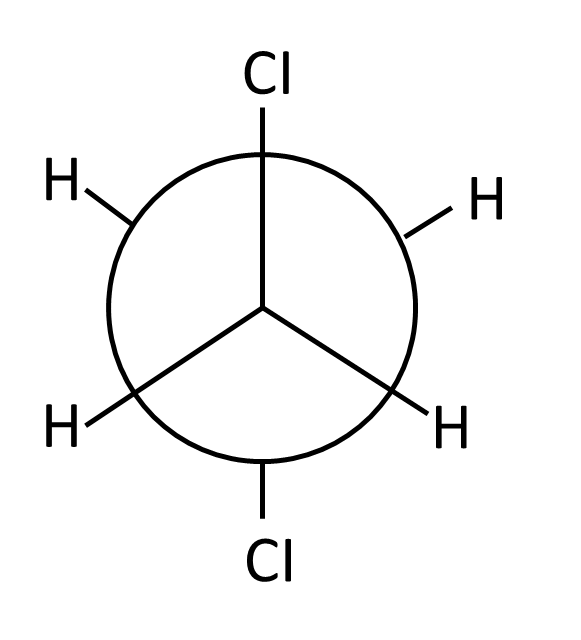
3. How can understanding isomerism help in solving important questions in Hydrocarbons for Class 11?
Isomerism is crucial as many important questions require:
- Identifying structural isomers for a given formula
- Explaining differences in boiling/melting points due to chain branching
- Distinguishing between positional, chain, and functional isomers
4. What are common exam pitfalls students face in Class 11 Hydrocarbons important questions?
- Incorrect IUPAC nomenclature due to skipping parent chain rules
- Confusing reaction mechanisms (for example, mixing up Markovnikov and anti-Markovnikov additions)
- Forgetting special tests to distinguish similar compounds (methane vs ethene; alkene vs alkyne)
- Ignoring resonance effects in aromatic stability
- Neglecting to write balanced chemical equations for conversions
5. How are high-order/application-based questions framed for Hydrocarbons in CBSE Class 11 exams?
These questions often ask students to:
- Predict products or explain observations based on molecular structure or conditions
- Distinguish mechanisms given similar reactants but different catalysts
- Relate structure and hybridization to reactivity or acidity order (e.g., ethyne vs ethene)
- Analyze aromaticity using Huckel's rule or predict resonance structures
6. What are the marking trends for Hydrocarbons Chapter 9 in CBSE Class 11 Chemistry (2025–26)?
- Short answer (1–2 marks): Nomenclature, definitions, simple tests
- Medium (3–4 marks): Mechanisms, multi-step conversions, isomer listing
- Long/HOTS (5+ marks): Comparative reasoning, aromaticity, conversion cycles, resonance explanation
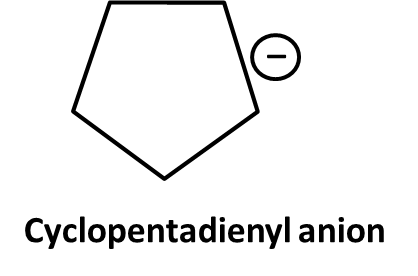
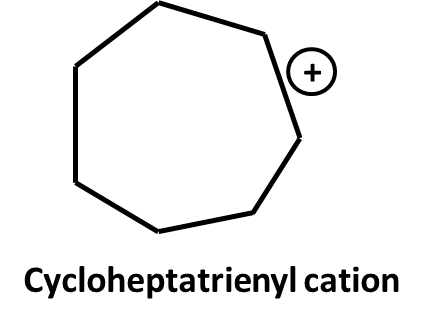
7. Why is aromaticity a frequently asked concept in Class 11 Hydrocarbons important questions?
Aromaticity is central because:
- It underpins the stability and reactivity of compounds like benzene, toluene, and aniline
- Exam questions often test you on Huckel’s Rule, delocalization, and resonance energy
- Distinguishing between aromatic, non-aromatic, and anti-aromatic is common in HOTS
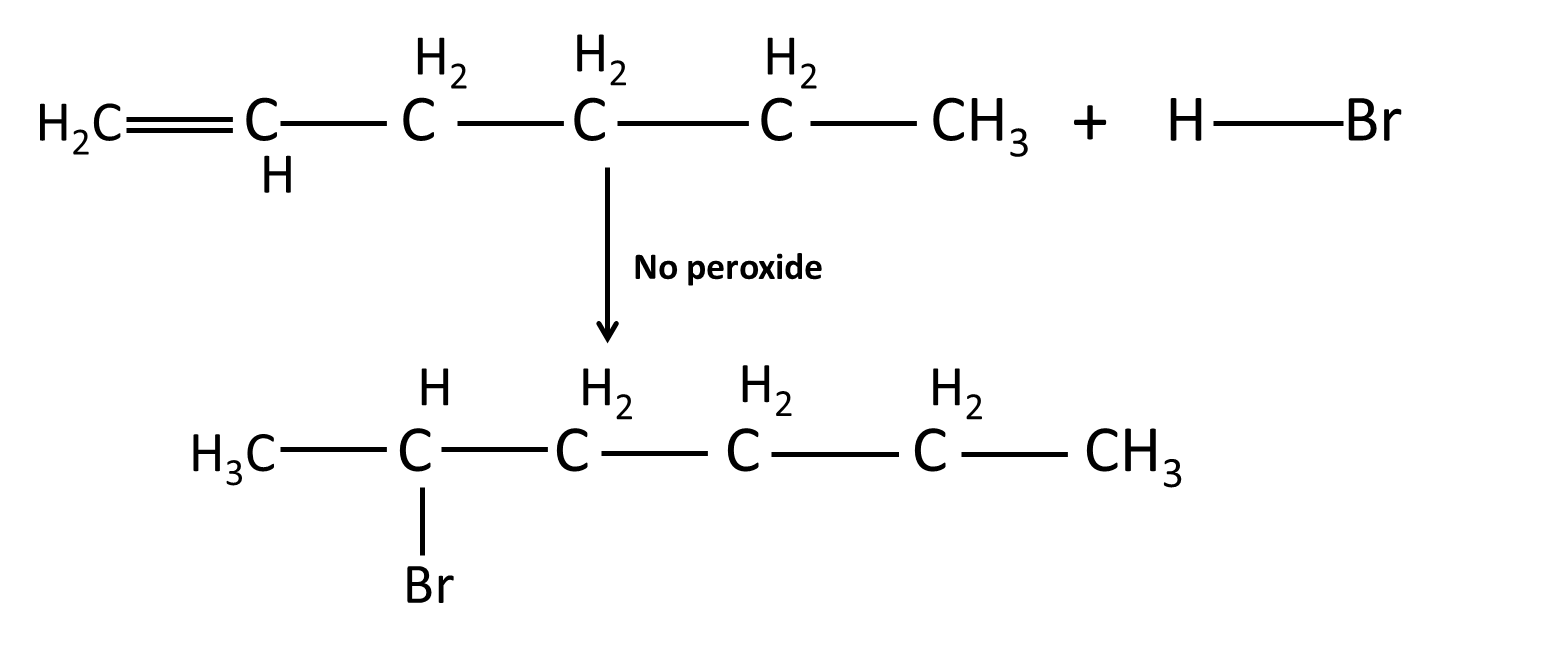
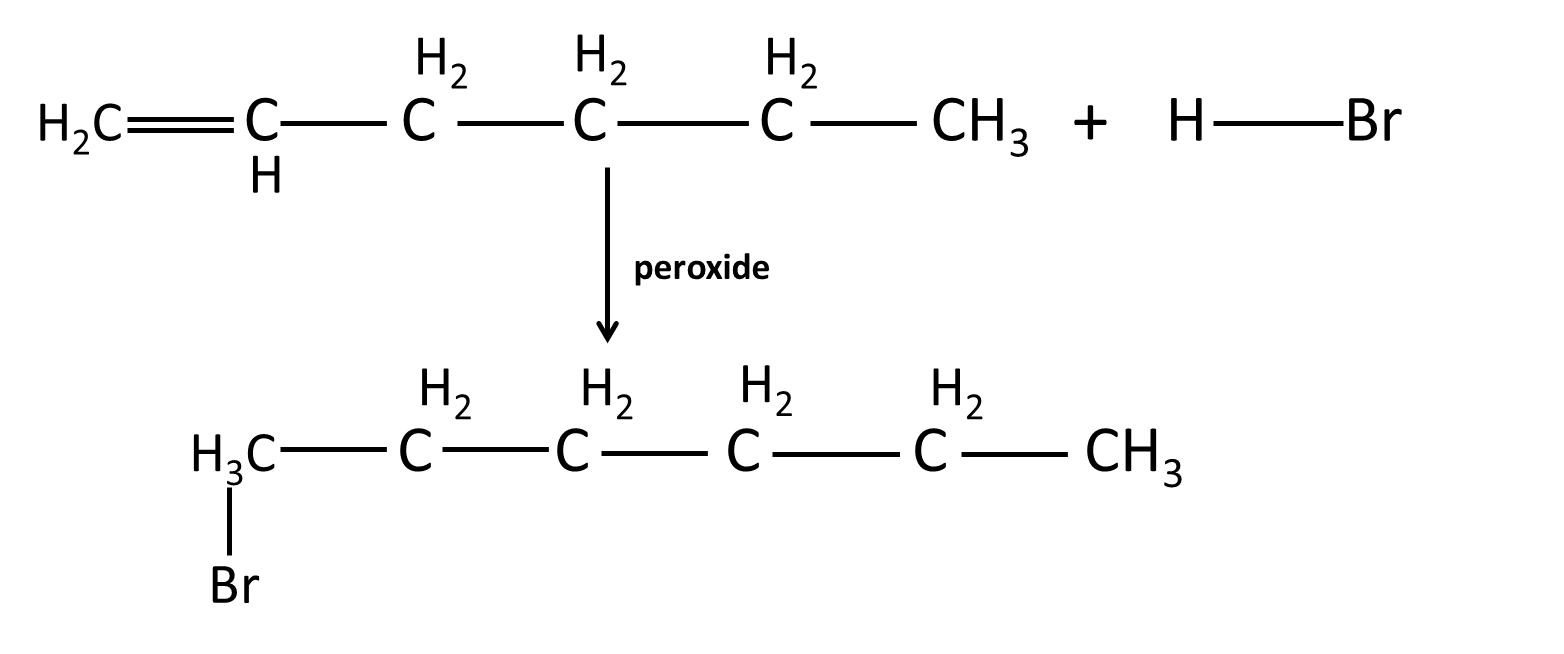
8. What are some misconceived ideas students have about Markovnikov and anti-Markovnikov additions in Hydrocarbons Chapter 9?
Common misconceptions include:
- Believing Markovnikov’s rule applies to all electrophilic additions — it does not when peroxides are present
- Confusing the direction of positive/negative species in addition to alkenes and alkynes
- Assuming alkynes always behave like alkenes in additions (reaction conditions matter)
9. How should students prioritize 5-mark and 3-mark questions while preparing for Hydrocarbons important questions?
Strategy includes:
- For 5-mark: Focus on multistep conversions, mechanism explanation (e.g., resonance in benzene, reactions of alkenes/alkynes), and comparative reasoning (e.g., stability, acidity order)
- For 3-mark: Practice structural isomer drawing, explaining results of distinguishing tests, and explaining boiling/melting point trends
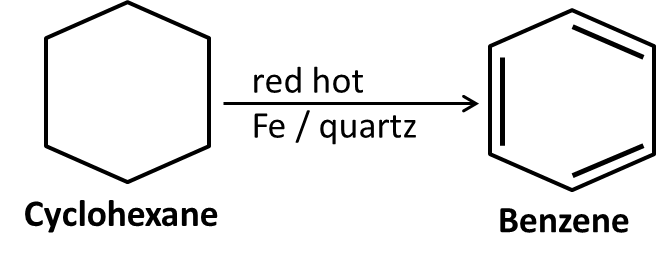
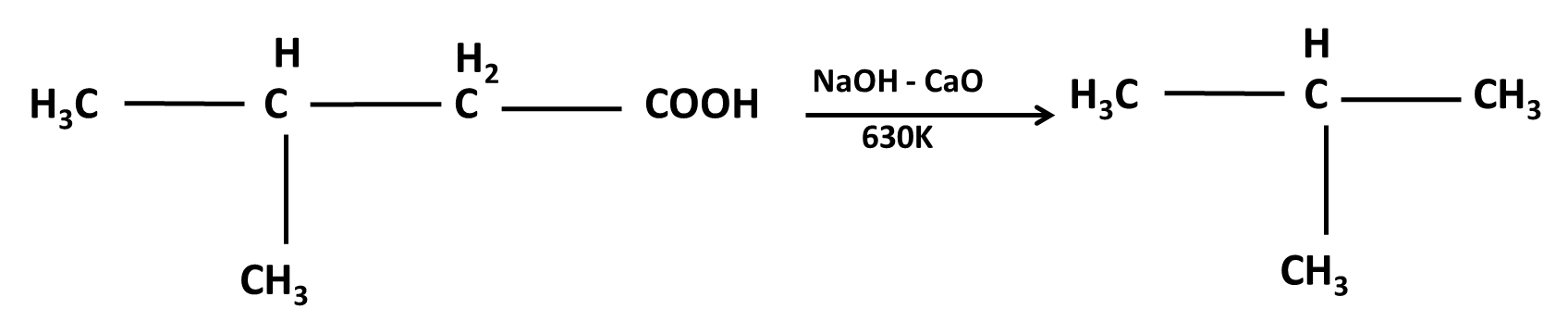
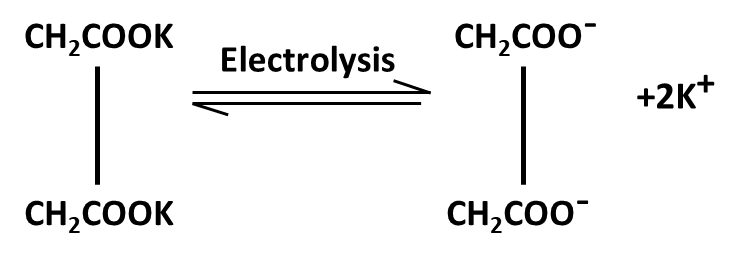
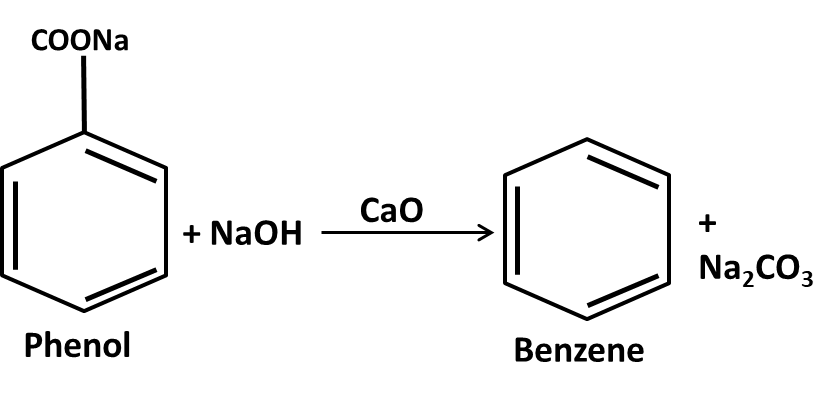
10. What role do named reactions (like Wurtz, Friedel–Crafts, Kolbe’s) play in Class 11 Hydrocarbons important questions?
Such reactions are commonly targeted in both direct and indirect formats:
- Questions may require writing balanced chemical equations or outlining the mechanism
- Contextual application: Using these in conversions between different types of hydrocarbons or for synthesis problems
- Explaining the conditions and possible outcomes if starting or end compounds change
11. How can I distinguish between alkanes, alkenes, and alkynes using chemical tests for CBSE Class 11 important questions?
- Alkanes: Generally unreactive; do not decolorize bromine water or Baeyer's reagent
- Alkenes/Alkynes: Decolorize bromine water (alkynes react more slowly)
- Baeyer's reagent (KMnO4): Turns colorless with alkenes/alkynes, no effect with alkanes
12. What are the key differences between resonance and aromaticity, and why do examiners emphasize them in Hydrocarbons important questions?
Resonance describes delocalization of electrons across atoms in a molecule, stabilizing the structure. Aromaticity is a special case where a cyclic, planar molecule exhibits continuous resonance, leading to extra stability (as in benzene).
- Exam questions test understanding by requiring explanations of why benzene is more stable than expected (due to resonance energy), or by asking which compounds fulfill aromaticity criteria (planarity, cyclic, delocalized electrons, Huckel number).
13. What strategies help solve Hydrocarbons Class 11 important questions with conversions and reaction sequences?
- Map out reagents and interconversions stepwise
- Memorize key reagents for preparation and transformation of alkanes, alkenes, alkynes, and aromatic compounds
- Pay attention to reaction conditions (e.g., presence/absence of peroxides, catalysts, temperature)
- Double-check the structures and IUPAC names at each step
14. Why is General Organic Chemistry (GOC) considered essential before solving Hydrocarbons Class 11 important questions?
GOC builds the foundation for understanding the behavior of hydrocarbons:
- It covers bonding, hybridization, electron movement, and intermediate types
- Questions often require knowledge of GOC for correctly predicting product formation, intermediate stability, and reactivity order
- CBSE and NEET/JEE questions interlink GOC with specific hydrocarbon concepts (e.g., acidity of alkynes)