




An Overview of Class 11 Biology Test For The Presence Of Albumin In The Urine Experiment
Albumin Protein: An Introduction
Albumin is a protein produced by the liver. This is mainly found in the blood and helps to keep fluid from leaking out of our blood vessels into other tissues. Albumin also carries hormones, vitamins, and enzymes in the blood. Without enough albumin, fluid can leak out of our blood and build up in our lungs, abdomen, or other body parts.
A trace amount of albumin is found in normal urine daily. The normal amount of albumin in the urine is less than 250 mg (in 24 hours urine sample).
Under pathological conditions, like Albuminuria, the albumin found in urine will be way above the normal level.
Table of Content
Aim
Theory
Material Required
Procedure
Observation
Result
Precaution
Aim
To experimentally test the presence of albumin in the urine.
Material Required
Urine sample
test tube
test tube holder
33% acetic acid
Bunsen burner
Dropper
Theory
The basic principle behind the heat coagulation test is the change in the protein’s structure because of high temperature and the change in pH. Heating a protein in an acidic condition causes the denaturation of proteins. Denaturation involves breaking weak hydrogen bonds responsible for proteins' secondary, tertiary, and quaternary structures; they become uncoiled and adhere to each other and form an insoluble mass.
In the test of heat coagulation of albumin. Heating cause denaturation of secondary, tertiary, and quaternary structure, only the primary structure remains intact, and the acetic acid added aids the breaking of the peptide bonds in the protein molecules, which facilitates the process of coagulation and adjust the pH at the isoelectric point of albumin which is 4.7.
Procedure
Step 1. Take a test tube, and hold it with a test tube holder.
Step 2. Fill two-thirds of the test tube with the urine sample.
Step 3. Incline the test tube at an angle and heat the upper one-third part of the tube in a low flame on a Bunsen burner.
Step 4. White cloudy precipitate develops in the heated one-third part of the urine sample.
Step 5. Add 1% acetic acid drop by drop, or simply add a drop of 33% acetic acid and boil.
Step 6. Wait and observe the appearance of the urine sample.
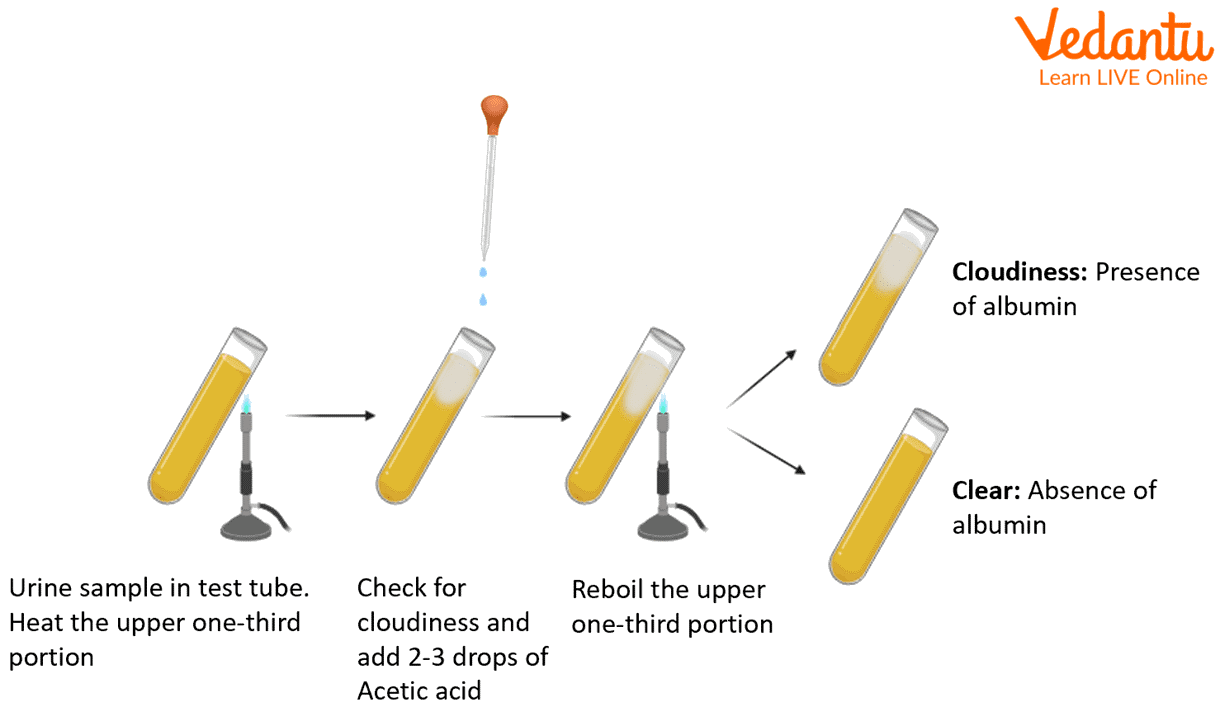
Test for the presence of albumin in the urine
Observation
After adding acetic acid and again boiling, if the turbidity appears and intensifies or remains, it indicates the presence of albumin in the urine.
Result
The positive outcome of heat coagulation is observed by the appearance of a turbid white precipitate on the top of the mixture. The lower portion of the solution acts as a control and compares the cloudiness that appears in the upper part.
Precautions
Clean the test tube before and after experimenting.
Handle the chemicals carefully.
Add acetic acid carefully, as the excess of acetic acid may dissolve traces of protein, giving a negative result.
Limitations
In certain situations, it could yield a positive result for other proteins
It is crucial to ensure the pH is within the range of the isoelectric pH of albumin.
Lab Manual Questions
1. What are the colour of the urine and the pigment responsible for this characteristic?
Ans: the normal colour of urine ranges from pale yellow to deep yellow colour. Urochrome pigment is responsible for this characteristic colour.
2. In which organ of our body highly toxic ammonia is converted into urea?
Ans: In the liver, ammonia is converted to a non-toxic compound called urea, which is then transported through the blood to the kidney.
3. Name the disorder in which glucose level is high in the urine.
Ans: Glucosuria is a condition in which there is a high level of glucose in urine.
4. Which reagent will demonstrate the presence of protein in urine?
Ans: Adding sulphosalicylic acid to the urine sample creates whitish-cloudy turbidity (coagulation) in the solution, indicating protein in the urine sample.
Viva Questions
1. What is the normal range of protein in urine?
Ans: normal range of protein in urine is less than 250 mg/ 24 hours sample.
2. What type of sample is preferred for urine protein tests?
Ans: early morning sample is preferred for testing the protein in the urine.
3. What are the different types of proteins found in urine?
Ans:
Albumin
Globulin
Mucin
Haemoglobins
4. What is the advantage of the heat coagulation test?
Ans: cheap test which does not require technical expertise.
5. What is the disadvantage of the heat coagulation test?
Ans: A specific type of protein cannot be specified.
6. Why should the test tube only heat on the upper one-third part?
Ans: urine in the lower portion of the test tube acts as a control and is to compare the cloudiness developed in the upper part of the test tube.
7. What is albuminuria?
Ans: albuminuria is a sign of kidney dysfunction. The level of albumin in the urine is much higher than the normal range.
8. What is the cause of albuminuria?
Ans: a damaged kidney lets some albumin pass into urine.
9. How to reduce albumin in urine?
Ans: to reduce albumin in urine, one can take medicines that lower the blood pressure prescribed by the physician.
10. What does acetic acid do in the heat coagulation test?
Ans: acetic acid added aids the breaking of the peptide bonds in the protein molecules, which facilitates the coagulation process and adjusts the pH at the isoelectric point of albumin, which is 4.7.
Practical Based Questions
What are the building blocks of protein?
DNA
RNA
Nucleotides
Amino acids
Ans: D) amino acids
What is a polypeptide having a molecular mass greater than 10,000 called?
Amino acids
Fats
Dipeptides
Proteins
Ans: D) proteins
Which of the proteins does not coagulate on heating?
Albumin
globulin
histone
mucoproteins
Ans: D) mucoproteins
Which of the following statements is valid for the primary structure of proteins?
The sequence of amino acids joined by weak hydrogen bonds
subunit of protein
a peptide bond joins amino acid sequences
form the helical structure of the protein
Ans: C) a peptide bond joins amino acid sequences
which protein structures are broken during the denaturation of protein
secondary structure
tertiary structure
primary structure
both a and b
Ans: D) both a and b
The isoelectric point of albumin?
5.6
4.7
2.6
9.4
Ans: B) 4.7
What causes a high level of albumin in the urine
Low blood pressure
Kidney dysfunction
High blood pressure
Both b and c
Ans: D) both b and c
If turbidity disappears in the heat coagulation experiment of urine, this indicates
Presence of fat
Presence of phosphate and carbonate
Presence of globulin protein
None of the above
Ans: D) presence of phosphate and carbonate
How to reduce albumin in urine?
Regular exercise
Medication
Proper diet
All of the above
Ans: D) all of the above
What is the bond of amino acids called?
Ionic bond
Covalent bond
Peptide bond
Hydrogen bond
Ans: C) peptide bond
Summary
A whitish cloudy turbid precipitate (coagulation) in the solution indicates the presence of albumin in the urine sample. A trace of albumin, which is less than 250 mg (in 24 hours urine sample), is found in normal urine. In kidney dysfunctions like albuminuria, albumin is found in urine above normal level.
FAQs on Class 11 Biology Test For The Presence Of Albumin In The Urine Experiment
1. How do you perform the test for the presence of albumin in urine for the Class 11 Biology practical exam (2025-26)?
This is a frequently asked practical question. The standard procedure is the Heat Coagulation Test:
- Take about 5 ml of the urine sample in a test tube.
- Heat the upper one-third portion of the test tube over a flame, keeping the lower part as a control.
- If a white, cloudy precipitate forms in the heated part, it indicates the presence of either albumin or phosphates.
- To confirm, add a few drops of 2% acetic acid. If the precipitate persists, it confirms the presence of albumin. If it dissolves, it was due to phosphates.
2. What specific observation confirms a positive result in the albumin test, and how is it distinguished from other substances?
A positive result for albumin is confirmed by the formation of a white, insoluble coagulum or precipitate in the upper heated portion of the urine sample that does not dissolve upon the addition of dilute acetic acid. This is a critical distinction because phosphates can also precipitate upon heating. However, phosphate precipitates will dissolve when acetic acid is added, while the coagulated albumin protein will not.
3. From an exam perspective, what are the key physiological conditions that can cause albumin to appear in urine?
The presence of albumin in urine, a condition called albuminuria, is an important diagnostic indicator. For your exam, you should know these key causes:
- Kidney Damage: The most significant cause is damage to the glomeruli of the nephrons. Damaged glomeruli become more permeable, allowing large protein molecules like albumin to leak from the blood into the filtrate.
- Pathological Conditions: Diseases such as glomerulonephritis, nephrotic syndrome, and high blood pressure can lead to albuminuria.
- Physiological Causes: In some non-pathological cases, temporary albuminuria can occur due to severe exercise, high fever, or pregnancy.
4. Why is the presence of albumin in urine considered a significant finding in a urinalysis exam?
This is a classic 'why' question. Albumin is a large protein that is normally retained in the bloodstream because the filtration slits of the glomeruli in a healthy kidney are too small for it to pass through. Its presence in urine (albuminuria) is significant because it is a primary indicator of kidney dysfunction or damage. It suggests that the kidney's filtration barrier has been compromised, which is a symptom of several underlying renal diseases. Therefore, this simple test is a crucial first step in diagnosing kidney-related health issues.
5. A student observes a white precipitate upon heating a urine sample, but it disappears after adding a few drops of acetic acid. What is the correct inference for the practical exam?
This is a Higher Order Thinking Skills (HOTS) type of question. The correct inference is that albumin is absent in the urine sample. The initial precipitate was due to the presence of phosphates, which are normally found in urine and can precipitate out upon heating. The fact that the precipitate dissolved upon adding acetic acid is the key step that confirms the absence of albumin and the presence of phosphates.
6. What are two essential precautions that are important to follow while performing the heat test for albumin?
To ensure accurate results in the practical exam, two important precautions are:
- Heating only the upper portion: Always heat only the upper one-third of the urine in the test tube. This allows the unheated lower portion to serve as a direct control, making it easy to compare and see the turbidity or precipitate.
- Handling acid carefully: Add the acetic acid drop by drop. Adding too much acid or adding it too quickly can sometimes dissolve a small amount of albumin, potentially leading to a false negative result.
7. For a 1-mark question, where is albumin synthesised, and why is it normally absent in a healthy person's urine?
Albumin is a protein synthesised in the liver. It is normally absent in urine because its molecular size is too large to pass through the semi-permeable membrane of the glomerular capillaries in the kidneys during the process of ultrafiltration.
























