




An Overview of Class 12 Chemistry Electrochemistry Experiment
Do you know what electrochemistry is? It is a branch of chemistry that converts chemical energy to electrical energy and vice versa. An electrochemical cell is a device that produces energy from electrochemical reactions. Electrochemical reactions occur in galvanic cells or Daniel cells, which are electrochemical cells.
There are two important cells of electrochemistry - the electrochemical cell and the electrolytic cell. The voltage of the cell changes with a change in the electrolyte concentration. To know more about this experiment, continue reading this article!!
Table of Content
Aim
Apparatus Required
Theory
Procedure
Observation
Result
Precautions
Aim
To study the variation of cell potential with a change in concentration of electrolyte at room temperature.
Apparatus Required
Following are some of the important apparatus required for this experiment
Glass beaker
Porous pot
Zinc strip
Copper strip
Voltmeter
0.1M ZnSO4
0.1 M CuSO4
Theory
Electrochemistry is the branch of chemistry that deals with the relationship between electrical changes and chemical changes. Electrochemical reactions are reactions that convert chemical energy into electrical energy, and it is a spontaneous reaction. These reactions occur in a galvanic cell. Here the zinc electrode will act as an anode, and the copper electrode will act as a cathode. On zinc electrodes, Oxidation takes place, and on copper electrodes, reduction takes place.
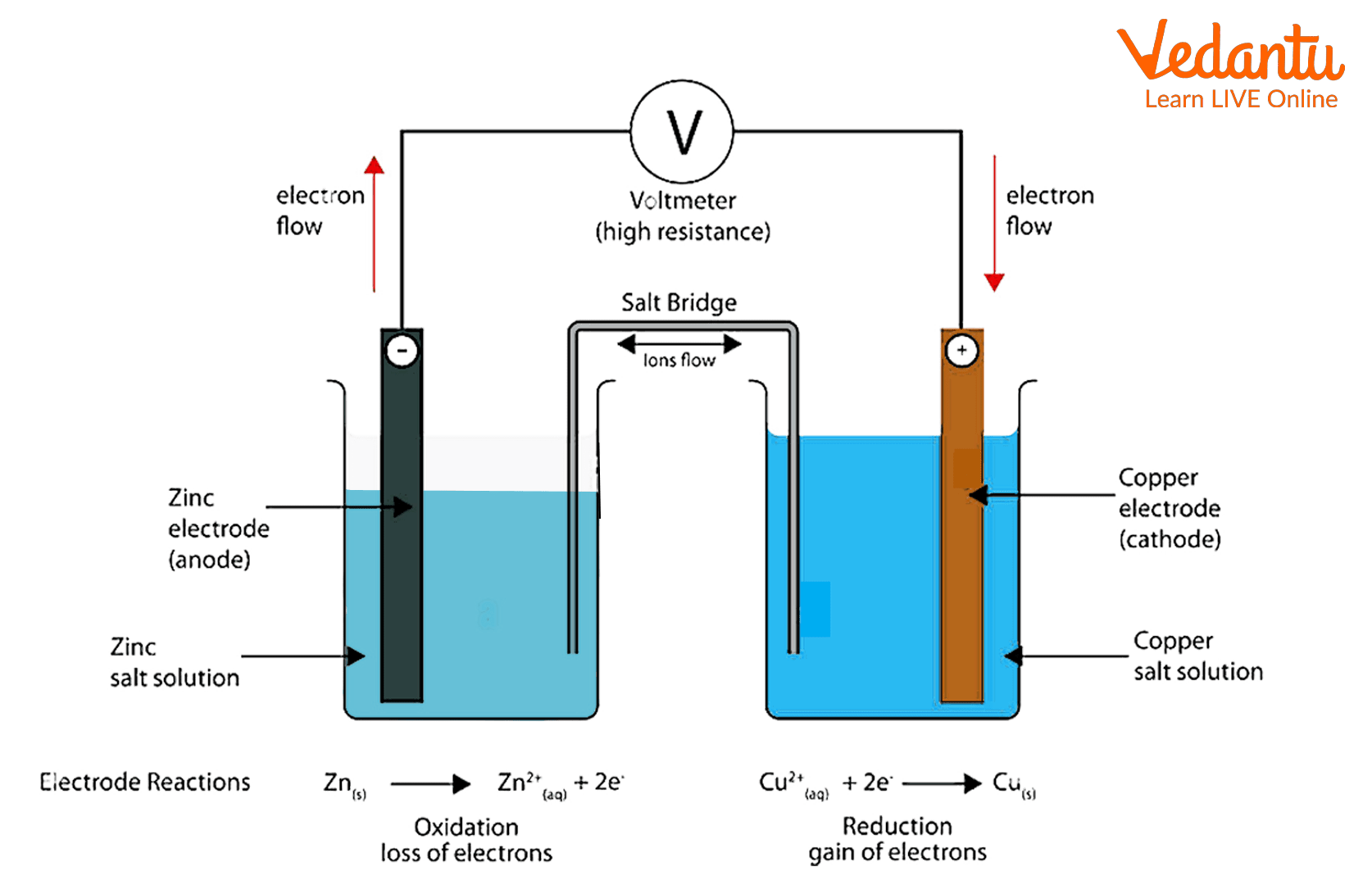
Daniel's cell
Procedure
The following are the important steps that need to be followed:
First, take the CuSO4 solution in a beaker and place a copper strip in it
Now add 100 ml zinc sulfate solution in a beaker and dip a zinc strip in it
Now connect the zinc strip with the negative terminal and the copper strip with the positive terminal of the voltmeter.
After connecting the strips with the voltmeter current starts to flow carefully, observing the reading of the voltmeter, which denotes the emf of the cell.
Repeat the above experiment by changing the concentration of zinc sulfate solution but keeping the concentration of copper sulfate the same.
Now again, repeat the above experiment by changing the concentration of copper sulfate and keeping the zinc sulfate concentration unchanged.
Observations
Results
The observed table and calculated emf from the first equation are the same.
The following formula can calculate Emf: Ecell = E°cell - RT logQ/nF
Where E is the emf of the cell,
E° is the standard emf of a cell
F is Faraday's constant.
Precautions
Following are some precautions that need to be followed while experimenting.
Copper and zinc strips must be cleaned with Sandpaper to remove dust
Reading of the voltmeter should be noted when the pointer becomes stable
The connections must be tight
Both the beaker and the porous pot must be washed and rinsed.
Lab Manual Questions
Q1 Define electromotive force.
Ans: Electromotive force is defined as the potential difference which leads to the flow of current from cathode to anode in an electrochemical cell.
Q2 What is oxidation?
Ans: Oxidation is defined as the process of losing an electron.
Q3 What is the difference between an electrochemical cell and an electrolytic cell?
Ans: In an electrochemical cell, chemical energy is converted to electrical energy, whereas in an electrolytic cell, electrical energy is converted to chemical energy.
Viva Questions
Q1 What is electrochemical series?
Ans: It is a series of elements arranged based on their increasing or decreasing standard reduction potential.
Q2 What is the importance of electrochemical series?
Ans: It helps us to know oxidising and reducing power of elements.
Q3 What do you mean by electrolysis
Ans: Electrolysis is converting electrical energy into chemical energy.
Q4 How can you define molar conductance?
Ans: Molar conductance is defined as the conduction of a one-mole solution placed between two parallel electrodes one centimetre apart.
Q5 What is kohlrausch law?
Ans: This law states that the limiting molar conductivity of an electrolyte is the sum of its cation and anion.
Q6 What is the difference between a primary battery and a secondary battery?
Ans: The primary battery is irreversible, whereas the secondary battery is a reversible type of battery.
Q7 How Can you define resistance?
Ans: Resistance is defined as the obstruction to current flow.
Q8 What is electrochemistry?
Ans: It deals with the study of the conversion of chemical energy to electrical energy.
Practical-Based Questions
Q1 In Which direction do electrons move in an electrochemical cell?
Cathode to anode
Anode to cathode
Both of the above
None of the above
Ans: 2. Anode to the cathode
Q2 Which of the following is the anode of Daniel's cell
Zinc rode
Copper rode
Both of the above
None of the above
Ans: 1. Zinc rode
Q3 Can we store copper sulphate solution in a zinc pot
Yes
No
May be stored
None of the above
Ans: 2. No
Q4 The value of equivalent conduction
Increases on dilution
Decreases on dilution
Remains the same on dilution
None of the above
Ans: 1. Increases in dilution
Q5 The value of specific conduction
Increases on dilution
Remains the same on dilution
Decreases on dilution
None of the above
Ans: 3. Decreases in dilution
Q6 Corrosion is an
Physical reaction
Chemical reaction
Electrochemical reaction
None of the above
Ans: 3. Electrochemical reaction
Q7 A species of higher standard reduction potential will
Undergo oxidation
Undergo reduction
Remain same
None of the above
Ans: 2. Undergo reduction
Q8 What is the standard reduction potential of the zinc electrode
0.37
0.76
0.6
0.56
Ans: 2. 0.76.
Conclusion
In this article, we have studied electrochemistry experiments and the electrochemistry formula.
We have also discussed the change in voltage on changing the concentration of the solution.
The steps of the experiment and articles required for conducting this experiment have also been discussed here.
Certain precautions which must be followed while experimenting have also been discussed.
FAQs on Class 12 Chemistry Electrochemistry Experiment
1. What types of numerical problems from the Electrochemistry chapter are most frequently asked in the Class 12 board exams?
For the CBSE Class 12 Chemistry board exam 2025-26, the most important numericals from Electrochemistry are typically based on:
- Nernst Equation: Calculating the EMF of a cell under non-standard conditions.
- Kohlrausch's Law: Determining the limiting molar conductivity (Λ°m) for weak electrolytes.
- Faraday's Laws of Electrolysis: Calculating the mass of a substance deposited or the amount of charge required.
- Relationship between ΔG° and E°cell: Calculating the standard Gibbs free energy change from the standard cell potential.
Practising these four types of problems is crucial for scoring well in the numerical section.
2. Which topics from Electrochemistry have the highest probability of appearing in the CBSE Class 12 Chemistry board exam for 2025-26?
Based on previous years' trends and syllabus weightage, the most expected topics for the 2025-26 board exam are:
- The Nernst equation and its applications, often as a 3-mark question.
- Faraday's laws of electrolysis, particularly the first law, with a numerical problem.
- The distinction between galvanic and electrolytic cells.
- Concepts related to conductance, including molar conductivity and the effect of dilution.
- Primary and secondary batteries, especially the lead storage battery and fuel cells, often appear in 2 or 3-mark conceptual questions.
3. How can a student score full marks on a 3-mark question involving the Nernst equation?
To secure full marks in a Nernst equation problem, ensure you follow these steps:
- Write the balanced cell reaction, clearly identifying the anode and cathode.
- Write the correct Nernst equation formula: Ecell = E°cell - (RT/nF) lnQ or Ecell = E°cell - (0.059/n) logQ at 298K.
- Correctly identify 'n', the number of electrons transferred in the balanced equation.
- Substitute all given values (E°cell, concentrations) correctly into the equation.
- Show the final calculation clearly and write the answer with the proper unit (Volts or V).
4. Why is the function of a salt bridge a frequently asked 2-mark question in Class 12 Chemistry exams?
The function of a salt bridge is a frequently asked question because it tests a fundamental concept of how a galvanic cell operates. Examiners favour this question to check if students understand that a cell cannot function without it. A complete answer must mention its two primary roles:
- It completes the electrical circuit by allowing the flow of ions between the two half-cells.
- It maintains electrical neutrality in the half-cells by supplying counter-ions to prevent the accumulation of positive and negative charges.
5. What is the key difference between a galvanic cell and an electrolytic cell, and what common mistakes should be avoided in exams?
The key difference is the type of energy conversion: a galvanic cell converts chemical energy into electrical energy through a spontaneous redox reaction, while an electrolytic cell uses electrical energy to drive a non-spontaneous chemical reaction.
Common mistakes to avoid:
- Confusing the sign of the electrodes: In a galvanic cell, the anode is negative and the cathode is positive. In an electrolytic cell, the anode is positive and the cathode is negative.
- Forgetting to state whether the reaction is spontaneous (galvanic) or non-spontaneous (electrolytic).
6. How is the concept of Gibbs free energy (ΔG°) related to the EMF of a cell, and why is this an important topic for board exams?
The relationship between standard Gibbs free energy (ΔG°) and the standard EMF of a cell (E°cell) is given by the equation: ΔG° = -nFE°cell. This equation is crucial because it connects thermodynamics with electrochemistry. It's an important exam topic because it helps determine the spontaneity of a redox reaction. A positive E°cell results in a negative ΔG°, indicating a spontaneous reaction, which is the basis for a functioning galvanic cell. This link between concepts is often tested in HOTS (Higher Order Thinking Skills) questions.
7. For a 5-mark question on Faraday's laws of electrolysis, what specific details and formulas must be included?
For a comprehensive 5-mark answer on Faraday's laws, you must include:
- First Law Statement: The mass of substance deposited at any electrode is directly proportional to the quantity of charge passed through the electrolyte. Formula: w ∝ Q or w = ZIt, where Z is the electrochemical equivalent.
- Second Law Statement: When the same quantity of charge is passed through different electrolytes connected in series, the masses of substances produced at the electrodes are directly proportional to their equivalent weights. Formula: w₁/w₂ = E₁/E₂.
- Definition of Terms: Clearly define w, I, t, Z, and E (equivalent weight).
- A simple numerical example or an application to demonstrate your understanding, if the question is theoretical.






































