




An Overview of Class 12 Chemistry Preparation Of Organic Compounds Viva Questions With Answers
What are organic compounds?
Ans. Carbon and hydrogen are covalently bound to one another and frequently to other elements to form organic molecules. Examples of organic compounds include benzoic acid, diethyl malonate, propanoic, butanoic, and malonic acids and aromatic and heterocyclic compounds.
What are functional groups?
Ans. An atom or group of atoms inside a molecule that exhibits a comparable set of chemical characteristics in various compounds is referred to as a functional group. The chemical characteristics of an organic compound are due to these functional groups.
What is Fehling’s solution?
Ans. Fehling's solution is made by combining two different solutions: Fehling's A, which is an aqueous potassium sodium tartrate solution that has been strongly alkalised with sodium hydroxide to produce a deep blue colour, and Fehling's B, which is aqueous potassium sodium tartrate solution that is also known as Rochelle salt and is colourless. Fehling's test is used to distinguish between reducing and non-reducing sugars. In the ketone functional community, this test can also be used to distinguish between solid and liquid carbohydrates.
What is Tollens Reagent?
Ans. Tollens' reagent is an alkaline solution of ammonium silver nitrate. It is a chemical reagent that can be used to differentiate between aldehydes and ketones, as well as some alpha-hydroxy ketones that can tautomerise into aldehydes.
What is an unsaturation test?
Ans. The double and triple bonds present in the organic compound can be determined using tests for unsaturation. The bromine test can be used to qualitatively determine if organic unsaturated hydrocarbons include phenols, anilines, or double or triple bonds between carbon atoms.
What is Benedict's Reagent?
Ans. The substance that is used in Benedict's test to identify simple sugars like glucose is known as Benedict's reagent. It is made by combining sodium citrate (Na₃C₆H₅O₇), copper sulphate pentahydrate (CuSO₄. 5H₂O), and sodium carbonate (Na₂CO₃) in distilled water. The solution is a vivid blue colour.
What is Rochelle’s salt?
Ans. It is a double salt of tartaric acid with the chemical formula C₄H₄O₆KNa·4H₂O. It is also referred to as potassium sodium tartrate or Rochelle salt. It has a taste that is cool and salty and is colourless to white crystalline powder.
Which test is performed to identify the presence of alpha-hydroxy ketone?
Ans. Aldehydes and ketones can be distinguished using the Tollens Test, a highly helpful technique. The silver mirror test is another name for this qualitative test. The Tollens test is often passed by compounds possessing an aldehydic group (aldehydes, alpha-hydroxy ketones and formic acid-its -COOH behaves like an aldehydic group).
Which Test is used to determine the presence of the carbonyl group?
Ans. Ketone or aldehyde functional group carbonyl functionality can be qualitatively identified using 2,4-Dinitrophenylhydrazine. Dinitrophenylhydrazone, a precipitate that might be yellow, orange, or red, forms when a test is positive.
What is Schmidt Reaction?
Ans. In the Schmidt reaction, hydrazoic acid and carboxylic acid combine in the presence of concentrated sulfuric acid to produce amine instantly.
From benzophenone and hydrogen azide, the Schmidt reaction can be utilised to make benzanilide. This is an example of how an amide can be created using a ketone and an azide.
$C_{2}H_{5}COOH+NH_{3}\overset{conc.H_{2}SO_{4}}{\rightarrow}C_{2}H_{5}NH_{2}+CO_{2}+N_{2}$
Which test is used to distinguish between Aldehydes and Ketones?
Ans. A qualitative laboratory test called the Tollens' test, commonly known as the silver-mirror test, is used to distinguish between an aldehyde and a ketone.
What is Schiff’s Reagent?
Ans. A substance called Schiff reagent is used in Schiff's aldehyde test. Aldehydes and Schiff reagents result in pink colour. It comprises sulfuric acid and fuchsin, also known as rosaniline hydrochloride.
What is the iupac name of benzanilide?
Ans. The preferred iupac name of benzanilide is N-Phenylbenzamide. It is also known by the name N-Benzoyl Phenylamine.
Why are Carboxylic groups more acidic than the phenolic group?
Ans. Due to resonance, the oxygen atom in carboxylic acids has a negative charge, but the less electronegative atom is the one with a negative charge in alcohols or phenols.
How will you distinguish phenol and aniline?
Ans. Carbylamine reaction can be used to distinguish between aniline and phenol. Phenol fails the test, but aniline yields a positive result. Aniline does not respond to this test, whereas phenol produces violet colour with neutral FeCl₃.
Which reagent is utilised to conduct the phthalein dye test?
Ans. The Phthalein dye test uses phthalic anhydride as a reagent. When an excessive amount of sodium hydroxide is added, a pink dye is produced, and the colour eventually fades.
What test may be used to determine whether the phenolic group is present?
Ans. The ferric chloride test is performed to determine whether phenols are present in a particular sample or compound (for instance, natural phenols in a plant extract).
What is Libermann’s test?
Ans. An intense green or blue colour results from the reaction of phenol with concentrated H₂SO₄ and NaNO₂, which, when diluted with water, turns red. The original green or blue colour is restored when the generated substance is present with NaOH or KOH. This process is named Liebermann's nitroso reaction.
Which substance was precipitated as a brown precipitate during the preparation of the ferric chloride solution?
Ans. Ferric hydroxide is the brown precipitate that forms during the production of ferric chloride solution.
How is the preparation of 2, 4, 6 Tribromoaniline carried out?
Ans. The preparation of 2, 4, 6 tribromoaniline from aniline is done by treating bromine water with aniline in a solution of acetic acid or diluted hydrochloric acid, a white precipitate immediately forms and that is 2,4,6-tribromoaniline.
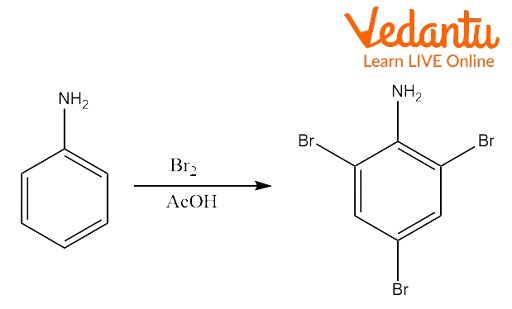
How does the treatment of phenol with bromine water affect the reaction?
Ans. When bromine water is added to phenol in a water solution, the phenol is discoloured, and a white precipitate with an antiseptic odour forms. 2,4,6-Tribromophenol is the precipitate.
What is the iupac name of acetanilide ?
Ans. The IUPAC Name is N-phenylacetamide.
A nitrogen atom is directly linked to a carbonyl in acetanilide, giving it an amide functional group (carbon-oxygen double bond).
What is Lucas’s reagent?
Ans. The Lucas test uses a solution of anhydrous zinc chloride in strong hydrochloric acid to distinguish and classify primary, secondary, and tertiary alcohols. This mixture is called Lucas reagent.
What is an iodoform test used for?
Ans. The iodoform test helps determine methanol and ethanol. Iodoform testing reveals a yellow precipitate for ethanol. Methanol, in contrast, does not pass the iodoform test.
Why is the sodium metal test performed before alcohol is dried?
Ans. Because sodium reacts violently with water, alcohol must be dried before a sodium metal test is performed.
Compared to the alcoholic group, the phenolic group is more acidic. Why?
Ans. Due to resonance stabilising the phenoxide ion, phenol is more acidic than alcohol. By stabilising the phenoxide ion, the presence of an electron-withdrawing group raises the acidity of phenol, whereas the presence of an electron-releasing group lowers the acidity of phenol by destabilising the phenoxide ion.
What is Baeyer’s reagent?
Ans. It is a cold potassium permanganate alkaline solution known as Baeyer's reagent (KMnO4). The qualitative organic analysis is used to check for unsaturation because it is a strong oxidising agent.
Do Benzene give Bromine water tests?
Ans. Benzene does not react with a bromine water solution despite having an unsaturated double bond and stable delocalised pi bonds.
Name a few tests used to check the unsaturation of organic molecules.
Ans. Two key tests for determining a compound's unsaturation are the bromine and Baeyer's tests. In Baeyer's test, the pink colour's disappearance denotes an unsaturated molecule. However, in the bromine test, the absence of the orange-red indicates an unsaturated molecule.
What are some of the tests that are used to identify primary, secondary, and tertiary amines?
Ans. The Hinsberg test is a chemical procedure frequently used to identify primary, secondary, and tertiary amines.
Which reagent is used in the Hinsberg test, and what is its name?
Ans. Benzene sulfonyl chloride is also known as the Hinsberg reagent. This name is known because it can be used in the Hinsberg test to identify and detect the primary, secondary, and tertiary amines in a given sample. Organosulfur compounds constitute this reagent.
When do we use the Isocyanide test?
Ans. Primary amines that are aromatic or aliphatic can perform an isocyanide test.
Why is an aniline a weaker base than ammonia?
Ans. It is difficult for the electrons on the N-atom of aromatic amines to transfer their electrons. Since the +I effect raises the electron density of the nitrogen atom, aniline is a weaker base than alkyl amines. This explains why aliphatic amine bases are more powerful than aromatic amine bases.
When do we use The dye test?
Ans. One can utilise the azo dye test to differentiate between aromatic and aliphatic amines. In this experiment, nitrous acid and amines react, forming the salt of diazonium. As a result of another aromatic amine attacking the aromatic amine's N₂ diazonium salt, which acts as an electrophile, N₂ is bridged between the two aromatic amines.
What is the Carbylamine test?
Ans. Another name for the carbylamine reaction is Hofmann's isocyanide test. With chloroform and alcoholic potassium hydroxide, the sample is heated in this experiment. The synthesis of isocyanide (carbylamine), which is readily recognised by its awful stench, will take place in the case of the presence of a primary amine.
What is the Azo-dye test?
Ans. The term "azo dye" refers to a broad category of synthetic organic dyes with nitrogen as an azo group or as part of their molecular structure (N=N). An example of an azo dye is Orange-1. The azo dye test is a method for identifying the presence of a primary aromatic amine group in an organic molecule.
What is the Ester test?
Ans. Alcohols and carboxylic acids react to create an ester with a fruity aroma. Esterification is the process that occurs when alcohol and carboxylic acid interact.
What is the diazotisation reaction?
Ans. It is known as "diazotisation" when a primary aromatic amine is chemically transformed into the equivalent diazonium salt of the amine.
What role does zinc dust play in the production of acetanilide ?
Ans. To stop aniline from oxidising during the process, zinc is added. It lessens the number of coloured impurities in the solution.
What is the Claisen-Schmidt reaction?
Ans. A ketone or aldehyde containing alpha-hydrogen combines with an aromatic carbonyl molecule that lacks alpha-hydrogens in a process known as the Claisen-Schmidt condensation reaction.
What is the IUPAC name of acetanilide?
Ans. Acetanilide is a solid compound with a leaf- or flake-like appearance that is odourless. N-Phenylacetamide is the IUPAC name for it.
What is the IUPAC name of benzanilide?
Ans. Benzanilide is a simple amide. It may be prepared by reacting benzoic acid and aniline directly. Its IUPAC name is N-Phenylbenzamide.
How will you prepare 2,4,6 tribromoaniline from aniline?
Ans. Even in the cold, aniline is nucleophilically substituted by bromine. In forming 2,4,6-tribromoaniline, the bromine atoms enter the two ortho locations and the para position. When bromine water is introduced to aniline, the bromine water is discoloured, and a white precipitate of 2,4,6, tribromo aniline is formed.
What is the Gatterrman Reaction?
Ans. The Gattermann reaction is a mechanism for formulating molecules with aromatic rings. Gattermann formylation and Gattermann salicylaldehyde synthesis are other names for the same process. The Friedel-Crafts reaction and the Gattermann reaction are similar.
FAQs on Class 12 Chemistry Preparation Of Organic Compounds Viva Questions With Answers
1. What are the most important board exam questions on the preparation of organic compounds for Class 12 Chemistry?
- Describe the methods for synthesising common organic compounds like ethanol, acetic acid, and benzene.
- Explain the mechanism and application of nucleophilic substitution reactions in organic synthesis.
- Discuss the significance of purification techniques such as crystallisation and distillation in organic chemistry practicals.
- Predict the product of a Grignard reagent reaction with a given aldehyde or ketone.
2. Which reactions are considered high-weightage in the preparation of organic compounds for CBSE 2025–26 Chemistry boards?
- Preparation of alcohols using reduction and hydrolysis methods
- Conversion of hydrocarbons to functionalised derivatives (e.g., alkane to alkyl halide)
- Reactions involving carboxylic acids (oxidation, reduction, esterification)
- Electrophilic substitution reactions in aromatic compounds
3. What are the common conceptual traps students face while preparing organic compounds for the board viva?
- Confusing isomerism types such as chain, positional, and functional isomerism
- Not distinguishing between addition and substitution reactions
- Ignoring the importance of reaction conditions (temperature, catalyst, solvent)
- Forgetting to balance chemical equations and account for byproducts
4. How can students effectively prepare for expected viva questions on organic compound synthesis in CBSE practical examinations?
- Study key reaction mechanisms and be able to draw stepwise intermediates
- Memorise important reagents and their roles in organic preparations
- Practice explaining experimental procedures and safety considerations
- Review previous year’s board viva trends for frequently repeated questions
5. Why is understanding the principle behind each organic compound preparation crucial for scoring full marks?
Grasping the underlying principle of each procedure ensures that students can logically predict outcomes, handle troubleshooting questions during viva, and explain the choice of reagents or conditions, all of which are assessed for full marks in exams.
6. What marking scheme is followed for questions on preparation and purification of organic compounds in the CBSE practical exams?
Generally, board marking weightage for organic compound preparation is as follows:
- Procedure and Principle Explanation: 2–3 marks
- Correct Reaction Equation: 2 marks
- Observation and Result: 1–2 marks
- Precautions and Viva: 2–3 marks
7. How can students distinguish between similar-sounding reagents in organic synthesis important questions?
Focus on the specific function and application of each reagent (e.g., LiAlH4 for strong reduction vs. NaBH4 for mild reduction) and refer to comparative tables provided in the syllabus to avoid confusion during viva or written answers.
8. What are some common board-level misconceptions regarding the synthesis of aromatic compounds?
- Assuming all aromatic ring reactions are electrophilic substitution—while some can proceed via nucleophilic or free radical mechanisms.
- Thinking that substituent effects are always activating or deactivating in the same manner across all reactions.
9. How does the CBSE board select important questions from the chapter 'Preparation of Organic Compounds' for board exams?
Selection is based on syllabus relevance, weightage trends over recent years, conceptual depth, and real-world applications. Priority is given to reactions that illustrate key concepts or are frequently used in practical/laboratory settings.
10. In what ways can students link the practical preparation of organic compounds to theoretical concepts for scoring high in both theory and viva?
Students should interconnect the theoretical background (like mechanisms and type of reactions) with actual practical steps performed in the lab, cite real experiment observations, and explain outcomes using the underlying chemistry. This approach demonstrates comprehensive understanding and maximises marks in both practical and theory sections.
























