




An Overview of Class 12 Chemistry Surface Chemistry Experiment
Surface chemistry deals with studying chemical changes at the interface of two phases. Colloids are a mixture of two substances, one consisting of dispersed insoluble particles suspended in another. The substance which is suspended is called the dispersed phase, and the one in which it is suspended is called the dispersion medium.
For example, a colloidal solution of starch (dispersed phase) in water (dispersion medium).
Table of Content
Aim
Theory
Procedure
Result
Aim
To prepare a colloidal solution of starch in water.
Apparatus Required
50ml beaker
250ml beaker
Mortar and pestle
Glass rod
Funnel
Filter paper
Reagents Required
Soluble starch
Distilled water
Theory
Based on the type of interaction between the dispersed phase and its dispersion medium, colloids are of two types -
Lyophilic sols-Where dispersion phase has a strong attraction towards the dispersion medium. They are highly solvated and stable. For example, starch sol.
Lyophobic sols-Where the dispersion phase has very little or no attraction towards the dispersion medium. They are less stable. For example, ferric hydroxide sol.
Starch, when heated with water, forms a stable lyophilic sol, due to hydrogen bonding between the OH group of starch and water molecules. This causes the starch particles to be highly solvated in water. The boiling condition of the water is necessary, as starch is insoluble in cold water.
Procedure
Weigh 0.5g of soluble starch, and transfer it to a mortar.
Add a few ml of distilled water (enough to make a thin paste) to the starch, and grind using a pestle, making it a thin paste. Transfer this paste to a 50ml beaker.
Take 100ml of distilled water in a 250ml beaker and bring it to a boil.
Once boiling is initiated, pour the starch paste using a glass rod, and stir continuously.
Boil the starch-water mixture for about 10 minutes, and then allow it to cool.
Once the solution is cooled to room temperature, filter it through a filter paper fixed in a funnel over a 250ml beaker. The filtrate is the lyophilic starch sol.
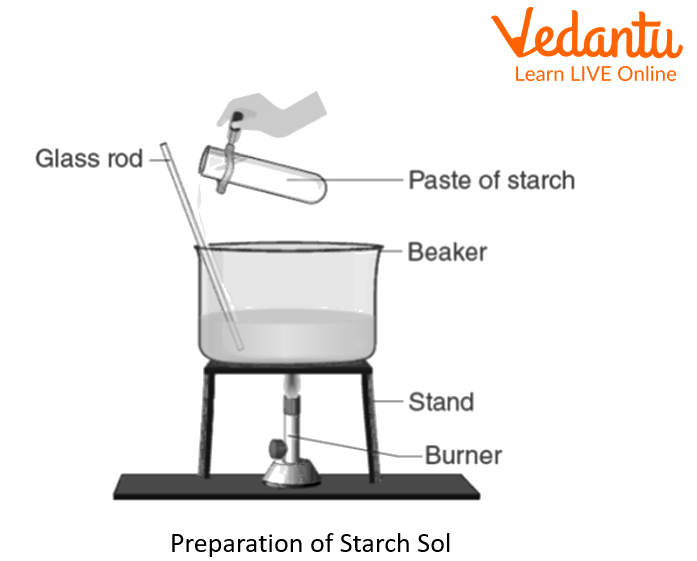
Preparation of Starch Sol
Result
Following the above-mentioned procedure, a colloidal solution of starch in water was successfully prepared.
Precaution
All the apparatus should be cleaned before use.
Starch paste should not be lumpy.
Starch paste should be added slowly using a glass rod, to avoid precipitation.
The boiling solution must be stirred continuously.
Lab Manual Questions
1. What is starch?
Ans. Starch is a carbohydrate with the formula (C6H10O5)n
2. What is lyophilic sol?
Ans. Lyophilic sols are solutions in which the solute has a great affinity for the solvent.
3. What is lyophobic sol?
Ans. Lyophobic sols are solutions in which the solute has very little or no affinity for the solvent.
4. Give one example of a lyophobic sol.
Ans. Ferric hydroxide sol
Viva Questions
1. State the importance of surface chemistry.
Ans. It helps us study chemistry at the surface level, i.e. at the interface of two phases.
2. What is the formula of starch?
Ans. (C6H10O5)n
3. What does lyophilic mean literally?
Ans. Solvent-loving
4. What does lyophobic mean literally?
Ans. Solvent fearing
5. Why is starch sol stable?
Ans. Due to strong hydrogen bonding between the OH group of starch and water
6. Gelatin powder is mixed in water. What is the dispersion medium here?
Ans. Water
7. Why does the starch paste need to add slowly?
Ans. To avoid precipitation.
8. Give one example of a liquid-liquid colloid.
Ans. Milk
9. How are carbohydrates important in the human body’s metabolism?
Ans. Carbohydrates break down into glucose, which is then used to produce
ATP.
10. What happens when starch is added to cold water?
Ans. Starch is almost insoluble in cold water.
Practical Based questions
Starch is a
Protein
Carbohydrate
Nucleic acid
Lipid
Ans: B)
Surface chemistry is the study of chemistry at _____ level
nascent
interface
endpoint
None of the above
Ans: B)
An emulsion consists of
liquid-solid
liquid-gas
liquid-liquid
solid-solid
Ans: C)
Which statement is not true about lyophobic sol?
They are less stable
They have high solvation
Ferric hydroxide sol is a lyophobic sol
None of the above
Ans: B)
Milk is an example of
emulsion
aerosol
foam
None of the above
Ans: A)
The dispersed phase in starch sol is
starch
water vapour
water
None of the above
Ans: A)
The formula of starch is
(C6N10O5)n
(C6H10O5)n
(C6H10O15)n
(C6N10O15)n
Ans: B)
Weak interaction between the dispersed phase and dispersion medium is shown in
Lyophobic sol
Lyophilic sol
Hydrophilic sol
None of the above
Ans: A)
Rubber is a
solid
liquid
colloid
none of the above
Ans: C)
To prepare starch sol, water should be
cold
Lukewarm
boiling
None of the above
Ans: C)
Conclusion
Starch is a long-chain polymer made up of sugar molecules connected through glycosidic linkage.
Starch, when heated with a dispersion medium such as water, forms a colloidal solution.
Colloids are mixtures where microscopic insoluble particles of one substance are dispersed in another.
The Starch solution is stable in the dispersion medium and remains unaffected by the electrolytic impurity.
FAQs on Class 12 Chemistry Surface Chemistry Experiment
1. What are the most frequently asked 3-mark questions from the Surface Chemistry chapter for the CBSE 2025-26 board exam?
For 3-mark questions in Surface Chemistry, students should focus on explaining concepts with examples. The most expected questions are:
Differentiating between physisorption and chemisorption on at least three parameters like specificity, enthalpy, and nature of forces.
Explaining the mechanism of heterogeneous catalysis using the adsorption theory.
Describing the preparation of colloids using Bredig's Arc method or peptization with a neat, labelled diagram.
Explaining the cleansing action of soaps and detergents.
2. Which topics in Surface Chemistry are most important for 5-mark questions?
For 5-mark questions, focus on topics that allow for detailed explanation and diagrams. As per previous board trends, prepare these topics thoroughly:
Properties of Colloids: A combined question on Tyndall effect, Brownian movement, and Electrophoresis is very common.
Adsorption Isotherms: Detailed explanation of the Freundlich adsorption isotherm, including its mathematical expression, plot, and limitations.
Coagulation: Explaining the coagulation of sols and the Hardy-Schulze rule, often paired with a numerical problem comparing the coagulating power of different ions.
3. How should one answer questions on the difference between lyophilic and lyophobic sols to score full marks?
To score full marks, present the differences in a tabular format. Key points to include are:
Ease of Formation: Lyophilic sols are formed easily by mixing, while lyophobic sols require special methods.
Stability: Lyophilic sols are highly stable due to charge and solvation, whereas lyophobic sols are less stable and depend only on charge.
Reversibility: Lyophilic sols are reversible, while lyophobic sols are irreversible.
Nature: Mention the 'solvent-loving' nature of lyophilic sols versus the 'solvent-hating' nature of lyophobic sols.
Example: Always provide one example for each, like starch/gelatin for lyophilic and metal sulphides/hydroxides for lyophobic.
4. Why is the Hardy-Schulze rule a critical concept for solving application-based questions in the board exam?
The Hardy-Schulze rule is critical because it tests the practical application of colloid chemistry. It's not just about reciting the rule, but applying it. Questions often require you to:
Identify the charge of a given sol (e.g., ferric hydroxide is positive, arsenious sulphide is negative).
Determine which electrolyte will be most effective for coagulation by comparing the valency of the oppositely charged ions.
Arrange given electrolytes in increasing or decreasing order of their flocculating power.
Mastering this rule is key for solving questions related to water purification and stability of sols.
5. What types of MCQs and 1-mark questions are expected from the 'Catalysis' section of this chapter?
From the catalysis section, expect direct and conceptual 1-mark questions. Important areas for MCQs include:
Identifying the catalyst in well-known industrial processes (e.g., iron in Haber's process).
Differentiating between homogeneous and heterogeneous catalysis based on an example reaction.
Understanding the role of promoters and poisons in catalysis.
Questions on the definition and examples of shape-selective catalysis by zeolites.
6. How are the concepts of electrophoresis and electro-osmosis different, and why is this a favourite topic for HOTS questions?
This is a favourite for HOTS (Higher-Order Thinking Skills) questions because it tests a very subtle distinction. In electrophoresis, the colloidal particles are charged and move towards the oppositely charged electrode when an electric field is applied, while the dispersion medium is stationary. In electro-osmosis, the movement of colloidal particles is prevented, and instead, the dispersion medium itself moves in the electric field. Understanding this difference shows a deep grasp of the electrical properties of the colloidal system as a whole.
7. What is a common mistake students make when explaining emulsions and how to avoid it in the exam?
A common mistake is to not specify the type of emulsion and the role of the emulsifying agent. An emulsion is a colloid of two immiscible liquids. For full marks, you must:
Specify the type: Is it oil-in-water (O/W) like milk, or water-in-oil (W/O) like butter?
Always mention the emulsifying agent (or emulsifier), which is the third component that stabilises the emulsion (e.g., casein in milk).
Simply defining an emulsion as a mixture of two liquids is incomplete and will likely lose marks.
8. Which diagrams from Surface Chemistry are most important and carry high marks?
Diagrams are crucial for scoring well in this chapter. The most important ones to practise for the CBSE 2025-26 exam are:
Electrophoresis: A U-tube showing the movement of colloidal particles to an electrode.
Dialysis/Electrodialysis: A clear setup showing the parchment bag, impure sol, and flow of water/electrodes.
Bredig's Arc Method: An illustration of the dispersion bath, ice bath, and electric arc between metal electrodes.
Micelle Formation: A diagram showing the arrangement of stearate ions above and at the critical micelle concentration (CMC).
Always ensure your diagrams are neat, well-labelled, and accurately represent the process.






































