




An Overview of Class 9 Chemistry Mixture Compound Experiment
Introduction
A mixture is a substance made up of two or more elements that can be physically separated from one another. There are many types of mixtures everywhere around you, including air in the environment, a solution of water and salt, sugar and water, and various gases.
When two or more chemical elements are brought together, a compound is formed. There are numerous examples of compounds that are used frequently, such as sodium chloride, and calcium chloride.
Table of Content
Aim
Physical changes
Chemical changes
Mixtures
Compounds
Result
Aim
To prepare a mixture and a compound by combining iron filings and sulphur powder and to distinguish between the mixture and the compound based on their appearance, behaviour towards magnet and carbon disulphide.
Materials Required
Bunsen burner
Tripod Stand
Wire gauze
Test tube stand
Test tubes
Test tube holder
China dish
Watch glass
Magnet
Iron filings
Carbon disulphide
Sulphur powder
Theory
Physical Changes
A substance's chemical content is unaffected by physical changes; only its appearance is altered. A procedure that doesn't transform a substance into something else entirely. The freezing of water, melting of wax, boiling of water, etc. are some examples of physical change.
Chemical Changes
A substance undergoes chemical transformations, becoming a completely new substance with a new chemical formula. Chemical reactions are another name for chemical transformations which is a procedure that involves changing one material (or several substances) into another and creating or breaking interatomic connections.
Mixtures
A mixture is defined as a substance made up of two or more substances that are physically separable.
Compounds
A compound is a material that is produced when two or more chemical elements are joined. One of the many instances of a substance that is often used is sodium chloride.
The compound formation is a chemical change as opposed to mixture creation, which is a physical change. In this experiment, mixtures and compounds will behave differently to magnet and carbon disulphide.
Procedure
1. Preparing a mixture of iron filings and sulphur powder
First, we properly mixed some amount of sulphur powder with iron filings.
Thus, a Mixture of sulphur powder and iron filings was the result.
Then we keep the Mixture formed in a china dish.
Bring a magnet to a mixture of iron filings and sulphur powder in a China dish.
After that, pour the mixture from the china dish into a test tube containing carbon disulphide.
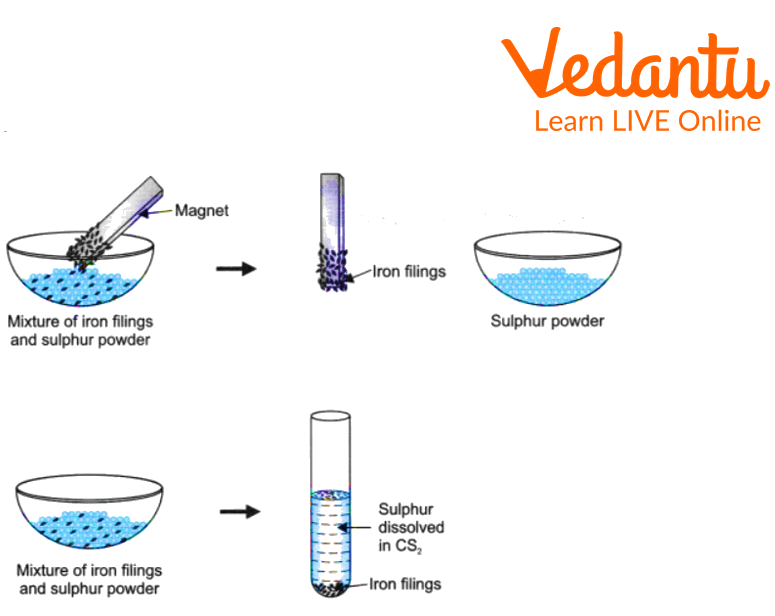
Mixture Preparation
2. Making a compound out of iron filings and sulphur powder
First, we placed a small amount of iron filings and sulphur powder in a test tube.
The mixture was then heated over a flame.
The iron sulphide compound was formed as a result of a reaction between iron and sulphur on heating.
The compound is to be kept on a china dish.
Bring a magnet to the compound in the china dish and observe what happens.
Then pour the compound into a test tube containing carbon disulphide and note down the observation.
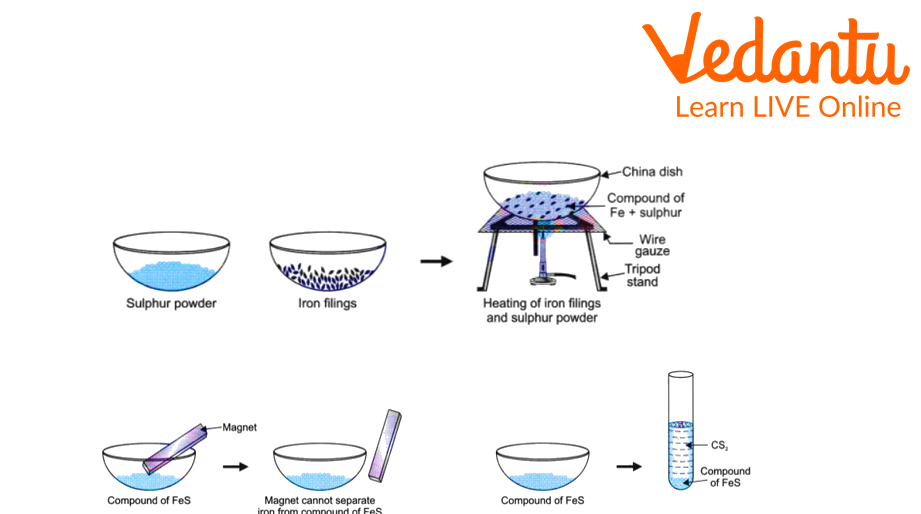
Compound Preparation
Observation
Result
Mixtures are prepared by mixing iron filings and sulphur powder, which is heterogeneous, can be separated by magnetic separation and does not behave towards carbon disulphide.
Compounds are formed by heating iron filings and sulphur powder which are homogeneous, can not be separated by the magnet and react with carbon sulphide.
Precautions
In a china dish or a hard glass, warm the mixture of iron filings and sulphur powder.
Don't breathe in any of the experiment's emitted gas.
Avoid exposing carbon disulphide to flame.
Lab manual questions
1. How can mixtures be separated?
Ans: Depending on the characteristics of the constituents, mixtures can be separated using a variety of physical techniques, such as filtering, decantation, evaporation, and magnetic separation.
2. Why can a mixture of sulphur and iron be separated using a magnet?
Ans: Iron, which is magnetic and retains its qualities in a mixture, is drawn to the magnet and can be separated from sulphur, which is not, by doing so.
3. Is mixing iron and sulphur a physical or chemical change?
Ans: It's a chemical change because both lose their properties.
4. What are different separating methods?
Ans:
Hand-picking.
Threshing.
Winnowing.
Sieving.
Evaporation.
Distillation.
Filtration or Sedimentation.
Separating funnel.
Viva questions
1. What are the different types of mixtures?
Ans: Mixtures are of two types based on their composition, Homogeneous and heterogeneous.
2. Give a few examples of mixtures.
Ans: Soda water, seawater and soil, air.
3. What is meant by physical change?
Ans: Physical changes are mainly reversible changes. No new product is formed in the physical changes.
4. Is rusting of iron a chemical change?
Ans: On rusting iron is converted into iron oxide. As a new product is formed it is a chemical change.
5. What is meant by compounds?
Ans: Compounds are substances made up of combining different elements in a particular ratio.
6. Is salt water a compound or a mixture?
Ans: Salt water is a homogeneous mixture where salt and water are in definite proportion.
7. Can magnetic separation apply to all mixtures?
Ans: No, only mixtures with magnetic properties, mainly metal, can be separated by magnetic separation.
8. Is water a mixture or a compound?
Ans: Because it is composed of molecules of hydrogen and oxygen, water is a compound. Water atoms don't exist. The ratio of two hydrogen atoms to one oxygen atom determines the composition of water molecules.
9. What are the different types of compounds?
Ans: Molecular, ionic and intermetallic, coordinate compound.
10. Give examples of a few compounds.
Ans: Table salt, carbon dioxide and cellulose, baking soda are examples of compounds.
Practical-based questions
1. What is the separation method for the iron and sulphur mixture?
Filtration
Magnetic separation
Decantation
Evaporation
Ans: (a) Magnetic separation. It is used to separate the mixture, iron powder will stick to the magnet.
2. What type of change occurs during the formation of compounds?
Physical change
Chemical change
No change
Both a & b
Ans: (B) Chemical Change. During the formation of a compound a new substance is formed, hence it is a chemical change.
3. Physical changes are generally _____.
Reversible
Irreversible
Both A) and B)
None of the above
Ans: (A) Reversible. A physical change is when a substance experiences a change in its physical characteristics. A physical change can usually be reversed.
4. Choose the compound from the following:
Crude oil
Seawater
Water
Ink
Ans: (C) Water. Water is a compound where ink, seawater, and crude oil are mixtures.
5. What are the methods to separate constituents from compounds?
Hand-picking
Filtration
Electrochemical method
Evaporation
Ans: (C) Electrochemical method. There are only two ways to separate a compound's constituents: chemically or electrochemically.
6. Which separation method is used for mixtures?
Evaporation
Filtration
Decantation
All of the above
Ans: (D) All of the above. Evaporation, filtration and decantation are common separation methods for mixtures.
7. Identify homogeneous mixtures.
Air
Oil in water
Vinegar
Both A) and C)
Ans: (D) Both (A) and (C). Air and vinegar are homogeneous mixtures having the same proportion of components.
8. Calcium chloride is an _____ compound.
Ionic
Molecular
Coordinate
Intermetallic
Ans: (A) Ionic. Calcium and chloride form an ionic bond, hence it is an ionic compound.
9. Choose the correct statement for heterogeneous mixtures.
They have the same composition
They have different compositions
Vinegar is a heterogeneous mixture
Both A) and C)
Ans: Heterogeneous mixtures have different compositions.
10. What are examples of chemical change?
Stretching of rubber
Cooking
Burning
Both C) and D)
Ans: Cooking and burning are chemical changes where new substances are formed.
Conclusion
From the preparation of the compound and mixture, we concluded that mixtures are heterogeneous and compounds are homogeneous substances. Mixture constituents particles can be separated physically by a magnet, while compounds can be separated only through chemical separation. Both compound and mixture react differently towards chemicals like carbon sulphide. Mixtures undergo physical changes, whereas compounds undergo chemical changes.
FAQs on Class 9 Chemistry Mixture Compound Experiment
1. What are the most important differences between a mixture and a compound that are often asked in exams?
For Class 9 exams, the key differences to remember between a mixture and a compound are:
- Composition: A mixture has a variable composition, while a compound always has a fixed composition by mass.
- Properties: In a mixture, the constituent substances retain their individual properties. In a compound, a new substance is formed with entirely new properties.
- Separation: Components of a mixture can be separated by physical methods (like filtration, magnetic separation). Constituents of a compound can only be separated by chemical or electrochemical reactions.
- Energy Change: No energy (heat, light) is usually evolved or absorbed during the formation of a mixture. Energy is typically released or absorbed during the formation of a compound.
2. When iron filings and sulphur powder are simply mixed, it is a physical change, but when the mixture is heated, it becomes a chemical change. Why is this a key concept for Class 9 exams?
This is a crucial higher-order thinking question because it tests the fundamental understanding of physical and chemical changes.
- When simply mixed, iron and sulphur form a mixture. The iron remains magnetic and the sulphur is soluble in carbon disulphide. No new substance is formed, so it's a physical change.
- When heated, the iron and sulphur react to form iron sulphide (FeS), a new compound. This compound is not magnetic, and its properties are completely different from its constituents. Since a new substance is formed, this is a chemical change. Understanding this distinction is vital for scoring well on application-based questions.
3. What are some important separation techniques for mixtures from Chapter 2, and what is the principle behind each?
Based on the CBSE 2025-26 syllabus, important separation techniques and their principles are:
- Evaporation: Used to separate a soluble solid from a liquid. The principle is that the liquid vaporises, leaving the solid behind.
- Centrifugation: Used to separate fine suspended particles from a liquid. The principle is that denser particles are forced to the bottom and lighter particles stay at the top when spun rapidly.
- Sublimation: Used to separate a component that sublimes (turns directly from solid to gas) from a non-sublimable one. The principle relies on the difference in volatility.
- Chromatography: Used to separate different solutes dissolved in the same solvent. The principle is based on the different rates at which components move through a stationary medium.
- Magnetic Separation: Used to separate a magnetic component from a non-magnetic one, based on the principle of magnetism.
4. Water is a compound, but salt water is a mixture. Why is this distinction crucial for understanding the properties of substances?
This distinction is crucial because it highlights the core definition of compounds and mixtures. Water (H₂O) is a compound because hydrogen and oxygen are chemically bonded in a fixed 2:1 ratio. The resulting molecule has properties (like its boiling point) that are completely different from hydrogen and oxygen gas. Salt water is a mixture because salt (NaCl) and water (H₂O) are only physically combined. Their ratio can vary, and both substances retain their original properties, which allows us to separate them by physical means like evaporation.
5. What are solutions, suspensions, and colloids? State one important characteristic to distinguish them, as expected in 3-mark questions.
For a 3-mark question, you should define and distinguish them as follows:
- A solution is a homogeneous mixture where solute particles are extremely small (less than 1 nm) and do not scatter light. Key characteristic: It is transparent and stable.
- A suspension is a heterogeneous mixture where solute particles are large (greater than 1000 nm), visible, and settle down over time. Key characteristic: It is unstable, and its particles settle out.
- A colloid is a type of mixture where particle size is intermediate (between 1 and 1000 nm). Key characteristic: It appears homogeneous but is actually heterogeneous and exhibits the Tyndall effect (scatters light).
6. From an exam perspective, what are some important examples of chemical and physical changes you should know for Class 9 Chemistry?
For exams, it's important to be able to classify everyday phenomena. Key examples include:
- Important Physical Changes: Melting of ice, boiling of water, dissolving sugar in water, tearing paper, and stretching a rubber band. These are generally reversible.
- Important Chemical Changes: Burning of wood or a candle, rusting of iron, cooking of food, and digestion. These involve the formation of new substances and are generally irreversible.
7. Most students can define homogeneous and heterogeneous mixtures, but what is a common misconception or trap question related to them in exams?
A common trap question involves alloys and colloids. Students often mistake an alloy, like brass (a mix of copper and zinc), for a compound because it looks uniform and has unique properties. However, it is a homogeneous mixture because its components are not in a fixed ratio and can be separated (though not always easily). Similarly, students might classify milk as homogeneous, but it is a colloid (a type of heterogeneous mixture) because its fat particles are suspended and scatter light.
8. What is the Tyndall effect, and which types of mixtures exhibit it? Why is this an important question for practical-based assessments?
The Tyndall effect is the phenomenon where a beam of light passing through a medium is scattered by the particles in it, making the path of the light visible. This effect is a key property of colloids and some fine suspensions. It is not shown by true solutions because their particles are too small to scatter light. This concept is important for practical assessments as it provides a simple visual test to distinguish a colloid from a true solution.
9. How does the concept of 'purity' in chemistry differ from its everyday meaning? Explain with reference to the chapter 'Is Matter Around Us Pure?'.
This is a high-level conceptual question. In everyday language, 'pure' often means free from adulteration, like 'pure milk' or 'pure ghee'. However, in chemistry, these are mixtures. A scientifically pure substance consists of only one type of particle (atoms or molecules). Therefore, elements (like iron) and compounds (like water or sugar) are considered pure substances, while milk, soil, and air are mixtures, regardless of their everyday 'purity'.
10. Based on the CBSE 2025-26 pattern, what are some important concepts from 'Mixtures and Compounds' that frequently appear as Multiple Choice Questions (MCQs)?
For acing the MCQ section, focus on these concepts:
- Identifying a substance as an element, compound, or mixture (e.g., classifying air, water, salt, and steel).
- Classifying mixtures as homogeneous or heterogeneous (e.g., soda water vs. muddy water).
- Recognising examples of colloids and their type (e.g., fog is an aerosol, milk is an emulsion).
- The specific principle behind a separation technique (e.g., chromatography is based on differential adsorption).
- Distinguishing between physical and chemical changes from a list of examples.





































