Kinetic Theory Class 11 important questions with answers PDF download
Free PDF download of Important Questions with solutions for CBSE Class 11 Physics Chapter 12 - Kinetic Theory prepared by expert Physics teachers from latest edition of CBSE(NCERT) books. Register online for Physics tuition on Vedantu.com to score more marks in your Examination.
Study Important Questions for class 11 physics Chapter 12 - Kinetic Theory
1 Marks Questions
1. Given Samples of $1\;{\text{c}}{{\text{m}}^3}$ of Hydrogen and $1\;{\text{c}}{{\text{m}}^3}$ of oxygen, both at N. T. P. which sample has a larger number of molecules?
Ans: Equal volumes of all gases, at equivalent temperatures and pressures, contain the same number of molecules, according to Avogadro's hypothesis. As a result, the number of molecules in both samples is the same. As a result, the number of molecules in both samples is the same.
2. Find out the ratio between most probable velocity, average velocity and root Mean Square Velocity of gas molecules?
Ans : Since,
Most Probable velocity, ${V_{mP}} = \sqrt {\dfrac{{2KT}}{m}} $
Average velocity, $\bar V = \sqrt {\dfrac{{8KT}}{{\pi m}}} $
Root Mean Square velocity: Vr.m.s. $ = \sqrt {\dfrac{{3KT}}{m}} $
Now, find out the Ratio between most probable velocity, average velocity and root mean square velocity of gas molecules
So, ${{\text{V}}_{ = p}}:\overline {\text{V}} {\text{Vrm}}.{\text{s}} = \sqrt {\dfrac{{2{\text{KT}}}}{m}} :\sqrt {\dfrac{{3KT}}{{\pi m}}} :\sqrt {\dfrac{{3{\text{KT}}}}{m}} $
$ = \sqrt 2 :\sqrt {\dfrac{8}{\pi }} :\sqrt 3 $
${{\text{V}}_{ = \rho }}:\overline {\text{V}} $ Vr.m.s. $ = 1.1.3:1.23$
3. What is Mean free path?
Ans: The average distance a molecule travels between collisions is known as the mean free path. It is symbolised by (lambda). Meters are the units of measurement (m).
4. What happens when an electric fan is switched on in a closed room?
Ans: When an electric fan is turned on, electrical energy is first transferred into mechanical energy, which is subsequently converted into heat. Heat energy increases the kinetic energy of air molecules, raising the temperature of the environment.
5. State the law of equi- Partition of energy?
Ans: The average kinetic energy of a molecule in each degree of freedom is equal to $\dfrac{1}{2}KT$. According to the law of equipartition of energy.
6. On what factors, does the average kinetic energy of gas molecules depend?
Ans: The absolute temperature is the only variable that affects average kinetic energy, and it is directly proportional to it.
7. Why the temperature less than absolute zero is not possible?
Ans: Since mean square velocity is proportional to temperature, it's a no-brainer. If the temperature is 0, the mean square velocity is also zero, and since molecules cannot be negative, temperatures lower than the absolute zone are not attainable.
8. What is the relation between pressure and kinetic energy of gas?
Ans: We are aware of this, Pressure$ = P$ Kinetic energy $ = {\text{E}}$
Now, Kinetic theory of gases is $P = \dfrac{1}{3}S{c^2} \to 1$ )
${\text{S}} = $ Density
$C = r.m.s$ Velocity of gas molecules
Translation’s mean kinetic energy per unit
Volume of the gas $ = E = \dfrac{1}{2}S{c^2} \to 2$ )
Dividing 1) by 2)
Now, we find out relation between pressure and kinetic energy of gas:
$\dfrac{P}{E} = \dfrac{{1S{c^2}2}}{{3 \times S{c^2}}} = \dfrac{2}{3}$
$ \Rightarrow P = \dfrac{2}{3}E$
9. What is an ideal perfect gas?
Ans: Ideal gas is defined as a gas that obeys the following laws or qualities.
1) The size of a gas molecule is zero.
2) There is no attraction or repellent force between gas molecules.
2 Marks Questions
1. If a certain mass of gas is heated first in a small vessel of volume ${{\text{V}}_1}$ and then in a large vessel of volume ${{\text{V}}_2}$. Draw the ${\text{P}}$ - T graph for two cases?
Ans: Equation of perfect gas; $P = \dfrac{{RT}}{V}$
Given, temperature is $P\alpha \dfrac{1}{V}$ As a result, when the gas is heated in a small vessel .
${V}_{1}$ the pressure rises faster than when the gas is heated in a big vessel (Volume).
${V}_{2}$ As a result, the slope of the graph in the case of a small vessel will be greater than in the case of a large vessel.
2. Derive the Boyle's law using kinetic theory of gases?
Ans: With the temperature constant, Boyle's law states that the volume of a given quantity of gas is inversely proportional to the pressure P, i.e. PV = constant.
The pressure exerted by a gas is now given by the kinetic theory of gases.
${\text{P}} = $ Pressure
${\text{V}} = $ Volume
$\overline {\text{V}} = $ Average Velocity
${\text{m}}$ = Mass of 1 molecule
${\text{N}} = $ No. of molecules
${\text{M}} = {\text{mN}}$ (Mass of gas)
$P = \dfrac{{1mN{{\bar V}^2}}}{{3V}}$
${P_V} = \dfrac{1}{3}M{\bar V^2}$
3. At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the r.m.s speed of a helium gas atom at- ${{20}^{0} }{\text{C}}$ ? Given Atomic Mass is ${\text{Ar}} = 39.9$ and ${\text{He}} = 4.0?$
Ans: Let we know that , Vr.m.s. and ${{\text{V}}^1}{\text{r}}$.m.s. are the root mean square speeds of Argon and helium
We have , Atoms at temperature ${\text{T}}$ and ${{\text{T}}^1}$ respectively.
${\text{R}} = $ Universal Gas constant
${\text{T}} = $ Temperature
${\text{M}}$ = Atomic Mass of Gas
Now, Vr.m.s. $ = \sqrt {\dfrac{{3RT}}{M}} $
${{\text{V}}^1}{\text{r}} \cdot {\text{m}}.{\text{s}}. = \sqrt {\dfrac{{3R{T^1}}}{{{M^1}}}} $
Given,${\text{M}} = $ Mass of Argon $ = 39.9$
${{\text{M}}^1} = $ Mass of Helium $ = 4.0$
${{\text{T}}^1} = $ Temperature of helium $ = - {20^0}{\text{C}}$
${{\text{T}}^1} = 273 + ( - 20) = 253\;{\text{K}}$
${\text{T}} = $ Temperature of Argon =?
Now, Vr.m.s. = ${{\text{V}}^1}{\text{r}} \cdot {\text{m}}.$
$\sqrt {\dfrac{{3RT}}{M}} = \sqrt {\dfrac{{3R{T^1}}}{{{M^1}}}} $
Squaring both side,
$\dfrac{{\not 3\,\not RT}}{M} = \dfrac{{\not 3\,\not R{T^1}}}{{{M^1}}}$
$\dfrac{T}{M} = \dfrac{{{T^1}}}{{{M^2}}} \Rightarrow T = \dfrac{{{T^1}M}}{{{M^1}}}$
Now it's time to put the numbers together.${T^1},{M^1}{\text{ \& }}M$
$T = \dfrac{{253 \times 39.9}}{{4.0}} = 2523.7\;{\text{K}}$
4. Show that constant - temperature bulk modulus ${\text{K}}$ of an ideal gas is the pressure ${\text{P}}$ of the gas?
Ans: When a substance P is exposed to increased pressure, a minor fractional volume drop occurs, which is related to bulk modulus k by:-
$K = \dfrac{{\Delta P}}{{\dfrac{{ - \Delta V}}{V}}} \to 1$)
So we know that A negative sign implies that the volume is decreasing. Before, compression in the case of an ideal gas at a constant temperature.
$PV = \dfrac{m}{M}RT \to 2$)
${\text{M}}$ = Molecular Mass of gas
We have, After compression at constant temperature,
$(P + \Delta P)(V + \Delta V) = \dfrac{m}{M}RT$
Now, From equation 2)
$PV = (P + \Delta P)(V + \Delta V)$
$P{V^\prime } = {P^\prime }{V^\prime } + P\Delta V + V\Delta P + \Delta P\Delta V$
or $ - P\Delta V = V\Delta P + \Delta P\Delta V$
$ - \dfrac{{P\Delta V}}{V} = \Delta P + \dfrac{{\Delta P\Delta V}}{V}(\because $ Dividing by ${\text{V}}$ on both sides )
$ - \dfrac{{P\Delta V}}{V} = \Delta P\left( {1 + \dfrac{{\Delta V}}{V}} \right)$
$ - \dfrac{{\Delta V}}{V} = \dfrac{{\Delta P}}{P}\left( {1 + \dfrac{{\Delta V}}{V}} \right)$
We're only concerned with minor fractional changes. Therefore, $\dfrac{{\Delta V}}{V}$ is much smaller than
1, As a result, it can be neglected as compared to 1.
$\therefore - \dfrac{{\Delta V}}{V} = \dfrac{{\Delta P}}{P}$
Using this value instead of $\dfrac{{\Delta V}}{V}$ in equation 1) we get
$K = \dfrac{{\Delta P}}{{\dfrac{{\Delta P}}{P}}} = P$
As a result, the bulk modulus of an ideal gas is equal to the gas's pressure under compression at constant temperature.
5. The earth without its atmosphere would be inhospitably cold. Explain Why?
Ans: Infrared radiation from the earth's surface is reflected back to the lower layers of the atmosphere. As a result, the earth's heat radiation from the sun is trapped by the atmosphere during the day. The earth's surface would become too cold to live if it didn't have an atmosphere.
6. If a vessel contains 1 mole of ${0_2}$ gas (molar mass 32 ) at temperature ${\mathbf{T}}$. The pressure of the gas is P. What is the pressure if an identical vessel contains 1 mole of Hit at a temperature $2{\text{T}}?$
Ans: According to the ideal gas equation: $ \to $
$PV = nRT$
$P = $ pressure
${\text{V}}$ =volume
${\text{n}} = {\text{No}}$. of molecule per unit volume
R=Universal Gas Constant
${\text{T}} = $ Temperature
So we get, $\dfrac{{PV}}{T} = nR\quad $ or $\dfrac{{PV}}{T} = $ constant
Hence $\left. {\dfrac{{{P_1}{V_1}}}{{{T_1}}} = \dfrac{{{P_2}{V_2}}}{{{T_2}}} \to 1} \right)$
Now, in response to the query,$ \to $
${P_1} = P\mid {T_1} = T$
${V_1} = V\mid {T_2} = 2T$
Using above equations in equation 1)
${P_2} = \dfrac{{{P_1}{V_1}}}{{{T_1}}}K\dfrac{{{T_2}}}{{{V_2}}}$
${P_2} = \dfrac{{PV}}{T}X\dfrac{{2T}}{V}\quad \;{{\text{V}}_1} = {{\text{V}}_2} = {\text{V}}(\because $ identical vessels )
${{\text{P}}_2} = 2{\text{P}}$
As a result, the pressure is doubled.
7. At very low pressure and high temperature, the real gas behaves like ideal gas. Why?
Ans: An ideal gas has a molecule volume of zero and no intermolecular forces.
1) At extremely low pressures, the amount of gas is so huge that the volume of a molecule is insignificant in comparison to the volume of gas.
2) Because the kinetic energy of molecules is very high at very high temperatures, the effect of intermolecular forces can be ignored.
As a result, at low pressure, real gases behave like an ideal gas.
8. Calculate the degree of freedom for monatomic, diatomic and triatomic gas?
Ans: The system's degrees of freedom are determined by:- ${\text{f}} = 3\;{\text{N}} - {\text{K}}$
Where, ${\text{f}} = $ degrees of freedom
${\text{N}} = $ Number of Particles in the system.
${\text{K}} = $ Independent relation among the particles.
Now ,
1) For a monatomic gas, ${\text{N}} = 1$ and ${\text{K}} = 0$
${\text{f}} = 3{\times }1 - 0 = 3$
2) For a diatomic gas; ${\text{N}} = 2$ and ${\text{K}} = {\text{m}}1$
${\text{f}} = 3{\times }2 - 1 = 5$
3) For a triatomic gas; ${\text{N}} = 3$ and ${\text{K}} = 3$
${\text{f}} = 3{\times }3 - 3$
${\text{f}} = 6$
As a result, the degrees of freedom for monatomic, diatomic, and Triatomic particles are 3, 5, and 6 respectively.
9. Determine the volume of 1 mole of any gas at s. T. P., assuming it behaves like an ideal gas?
Ans: Using the ideal gas equation-
${\text{P}} = $ Pressure
${\text{V}} = $ Volume
${\text{n}} = {\text{No}}$. of moles of gas
${\text{R}} = $ Universal Gas Constant
${\text{T}} = $ Temperature
We have,
${\text{PV}} = {\text{nRT}}$
${\text{V}} = \dfrac{{nRT}}{P}$
Given is , ${\text{n}} = 1$ mole; ${\text{R}} = 8.31\;{\text{J}}/{\text{mol}}/{\text{K}};{\text{T}} = 273\;{\text{K}}$
${\text{P}} = 1.01 \times {10^5}\;{\text{N}}\mid {{\text{m}}^2}$
${\text{V}} = \dfrac{{1 \times (8.31) \times 273}}{{1.01 \times {{10}^3}}}$
${\text{V}} = 22.4 \times {10^{ - 3}}\;{{\text{m}}^3}$
Since 1 litre
$ = 1000\;{\text{c}}{{\text{m}}^3}$
$ = 1 \times {10^{ - 3}}\;{{\text{m}}^3}$
Hence ${\text{V}} = 22.41$
i.e. at S.T.P., any gas has a volume of 22.41. (Standard Temperature & Pressure).
10. A tank of volume $0.3{m^3}$contains 2 moles of Helium gas at ${{20}^{0} }{\text{C}}$. Assuming the helium behave as an ideal gas;
Ans: 1) Find the total internal energy of the system.
Given, ${\text{n}} = $ No. of moles $ = 2$
${\text{T}} = $ Temperature $ = 273 + 20 = 293\;{\text{K}}$
${\text{R}} = $ Universal Gas constant $ = 8.31\;{\text{J}}/{\text{mole}}.$
So we know that, Total energy of the system $ = {\text{E}} = \dfrac{3}{2}nRT$
Hence, $E = \dfrac{3}{2} \times n \times 8.31 \times 293E = 7.30 \times {10^3}J$
2) Determine the r. m. s. Speed of the atoms.
Molecular Mass of helium $ = 4\;{\text{g}}$ \ mol
$ = \dfrac{{4 \times {{10}^{ - 3}}{\text{Kg}}}}{{mol}}$
Now , Root Mean speed $ = {\text{Vr}} \cdot {\text{m}} \cdot {\text{s}} = \sqrt {\dfrac{{3RT}}{M}} = \sqrt {\dfrac{{3X8.31 \times 293}}{{4X{{10}^{ - 3}}}}} $
Vr.m.s. $ = 1.35{\text{X}}{10^3}\;{\text{m}}/{\text{s}}$
11. State Graham's law of diffusion and derive it?
Ans: The rates of diffusion of two gases are inversely proportional to the square roots of their densities, according to Graham's law of diffusion.
Consider two gases diffusing into one another at a certain pressure. Let ${{\text{S}}_{\text{A}}}$ and ${{\text{S}}_{\text{B}}}$ be their densities.
So we know that, the root Mean square velocities of the molecules of gases A and B will be: $ \to $
$\left. {{{\text{V}}^{\text{A}}}{\text{r}} \cdot {\text{m}} \cdot {\text{s}} = \sqrt {\dfrac{{3P}}{{{S_A}}}} \to 1} \right)$
$\left. {{{\text{V}}^{\text{B}}}{\text{r}} \cdot {\text{m}} \cdot {\text{s}} = \sqrt {\dfrac{{3P}}{{{S_\mathbb{B}}}}} \to 2} \right)$
Now, Dividing equation 1) by 2)
$\left. {\dfrac{{{{\text{V}}^A}{\text{r}} \cdot {\text{m}} \cdot {\text{s}}.}}{{{{\text{V}}^{\text{B}}}{\text{r}} \cdot {\text{m}}.{\text{s}}}} = \sqrt {\dfrac{{3P}}{{{S_A}}}} X\sqrt {\dfrac{{{S_B}}}{{3P}}} \quad = \sqrt {\dfrac{{{S_B}}}{{{S_A}}}} \to 1} \right)$
The rate of diffusion of a gas is now directly related to its molecules' r.m.s. velocity. If and are the gas diffusion rates, and B is the rate of diffusion of gases, then
$\dfrac{{{r_A}}}{{{r_B}}} = \dfrac{{{V^A}rm.s}}{{{V^B}{r_M}.s}} = \sqrt {\dfrac{{{S_B}}}{{{S_A}}}} $
Or $ \Rightarrow $ this is Graham's law.
$\dfrac{{{r_A}}}{{{r_B}}} = \sqrt {\dfrac{{{S_B}}}{{{S_A}}}} $
12. State Charles's law? If air is filled in a vessel At ${{60}^{0}}{\text{c}}$. To what temperature should it be heated in order that $\dfrac{{{1^{rd}}}}{3}$ of air may escape out of the vessel?
Ans: According to Charles' law, the volume of a given mass of a gas is precisely proportional to its Kelvin temperature when pressure remains constant, i.e.
V & T if pressure is constant; ${\text{V}} = $ volume ${\text{T}} = $ Temperature
Or $\dfrac{V}{T} = $ constant
We have, ${{\text{T}}_1} = 60 + 273 = 333{\text{k}}$
${{\text{v}}_1} = {\text{V}}$
${{\text{T}}_2} = $ ?
${{\text{v}}_2} = {\text{V}} + \dfrac{V}{3} = \dfrac{4}{3}V$
So now , from Charles's show,
$\dfrac{{{V_1}}}{{{T_1}}} = \dfrac{{{V_2}}}{{{T_2}}} \Rightarrow \dfrac{{{V_1}}}{{{V_2}}} = \dfrac{{{T_1}}}{{{T_2}}}$
${{\text{T}}_2} = {{\text{T}}_1}\dfrac{{{V_2}}}{{{V_1}}}$
${{\text{T}}_2}\dfrac{{333 \times 4\;{\text{V}}}}{{3 \times V}}$
Hence,
${{\text{T}}_2} = {171^0}{\text{c}}$ or $444{\text{k}}$
13. Show that average kinetic energy of translation per molecule of gas is directly proportional to the absolute temperature of gas?
Ans: According to the kinetic theory of gases, one mole of an ideal gas exerts a pressure of
${\text{P}} = \dfrac{{1M{C^2}}}{{3\;{\text{V}}}}\quad {\text{M}} = $ Mass of gas
or ${\text{PV}} = \dfrac{{1M{C^2}}}{3}\;{\text{V}} = $ Volume of gas
Since ${\text{PV}} = {\text{RT}}$ (for 1mole of gas)
or $\dfrac{1}{3}M{C^2} = RT\quad $ R=Universal gas constant
${C^2} = \dfrac{{3RT}}{M}\quad \;{\text{T}} = $ Temperature
So Now, $C\alpha \sqrt T $
Also, $\dfrac{1}{3}M{C^2} = RT$
Dividing by number of molecules of gas $ = {\text{N}}$
$\dfrac{{1M}}{{3N}}{C^2} = \dfrac{R}{N}T$
${\text{K}} = $ Boltzman constant
$\dfrac{1}{3}m{c^2} = KT \to $ Dividing
or $\dfrac{1}{2}m{c^2} = \dfrac{3}{2}KT$
We get , $\dfrac{1}{2}m{c^2} = $ Kinetic energy per molecule of gas
Now, $\dfrac{1}{2}m{c^2}\alpha T$
as $\dfrac{3}{2}k = $ constant
14. Air pressure in a car tyre increases during driving? Why?
Ans: Because of the action, the temperature of the air inside the tyre rises during driving. According to Charles's law, as the temperature rises, the pressure inside the tyres rises as well.
15. Four molecules of gas have Speeds $2,4,6,8,\;{\text{km}}/{\text{s}}$. Respectively.
Ans: Here, ${{\text{C}}_1} = {\text{km}}/{\text{s}} = $ velocity of first gas
${{\text{C}}_2} = 4\;{\text{km}}/{\text{s}}$ l=velocity of second gas
${{\text{C}}_3} = 6\;{\text{km}}/{\text{s}} = $ velocity of third gas
${{\text{C}}_4} = 8\;{\text{km}}/{\text{s}}$ =velocity of fourth gas
1) Average speed -
Calculate the average period –
We Get used formula is,
$\therefore $ Average speed $ = \dfrac{{{C_1} + {C_2} + {C_3} + {C_4}}}{4}$
Now,
Average Speed $ = \dfrac{{2 + 4 + 6 + 8}}{4}$
i.e Average Speed $ = \dfrac{{20}}{4} = 5\;{\text{km}}/{\text{s}}$
2) Root Mean square speed -
Calculate the Root mean square speed –
We Get used formula is,
Root Mean Square Speed $ = \sqrt {\dfrac{{C_1^2 + C_2^2 + C_3^2 + C_4^2}}{4}} $
Now ,
R. m. s of gas$ = \sqrt {\dfrac{{{2^2} + {4^2} + {6^2} + {8^2}}}{4}} $
R. m. s of gas$ = \sqrt {\dfrac{{120}}{4}} $
i.e R. m. s of gas$ = 5.48km/s$
3 Marks Questions
1. If Nine particles have speeds of $5,8,12,12,12,14,14,17$and $20\;{\text{m}}/{\text{s}}$. Find.
Ans: 1) the average speed
Divide the total number of particles by the sum of their speeds to get the average speed.
Hence, Average speed, $\bar V = \dfrac{{5 + 8 + 12 + 12 + 12 + 14 + 14 + 17 + 20}}{9} = 12.7\;{\text{m}}\mid {\text{s}}$
2) the Most Probable speed
The average value of the square of speeds is given by:-
Hence,${\bar V^2} = \dfrac{{{5^2} + {8^2} + {{12}^2} + {{12}^2} + {{12}^2} + {{14}^2} + {{14}^2} + {{17}^2} + {{20}^2}}}{9}$
$ = \dfrac{{25 + 64 + 144 + 144 + 144 + 196 + 196 + 289 + 400}}{9} = \dfrac{{1602}}{9}$
i.e. ${\bar V^2} = 178\;{{\text{m}}^2}/{{\text{s}}^2}$
RM $S$ speed,$Vrm.s = \sqrt {{{\bar V}^2}} = \sqrt {178} = 13.3\;{\text{m}}/{\text{s}}$
3) the r.m.s. Speed of the particles
Three particles have a speed of, two particles have a speed of, and the remaining particles have varying speeds. As a result, the most likely speed,
${{\text{V}}_{{\text{mP}}}} = 12\;{\text{m}}/{\text{s}}$
2: Establish the relation between $Y\left( { = \dfrac{{{C_P}}}{{{C_V}}}} \right)$ and degrees of freedom (n)?
Ans: Now $y = \dfrac{{{C_p}}}{{{C_V}}}$
Where ${\text{CP}} = $ specific heat at constant pressure
${\text{CV}} = $ Specific heat at Constant volume.
and ${\text{n}} = $ Degrees of freedom $ \to $ is the total number of co-ordinates or independent quantities needed to completely describe the system's position and configuration.
Suppose, a polyatomic gas molecule has ' ${{\text{n}}^\prime }$ degrees of freedom.
$\therefore $ Total energy associated with a gram molecule of the gas i. e.
Here,
${\text{N}} = $ Total number of molecules
${\text{R}} = $ Universal Gas Constant
${\text{R}} = {\text{NK}}$
${\text{K}} = $ Boltzmann Constant
${\text{E}} = n \times \dfrac{1}{2}{\text{KT}} \times {\text{N}} = \dfrac{n}{2}RT$
So We know that,
Specific heat at constant volume,
${C_v} = \dfrac{{dE}}{{dT}}$
${C_V} = \dfrac{d}{{dT}}\left( {\dfrac{n}{2}RT} \right)$
${C_V} = \dfrac{n}{2}R$
Now Specific heat at constant Pressure, ${{\text{C}}_{\text{P}}} = {\text{Cv}} + {\text{R}}$
${C_P} = \dfrac{n}{2}R + R$
${C_P} = \left( {\dfrac{n}{2} + 1} \right)R$
As, $Y = \dfrac{{{C_p}}}{{{C_\nabla }}}$
$Y = \dfrac{{\left( {\dfrac{n}{2} + 1} \right)R}}{{\dfrac{n}{2}R}}$
$Y = \left( {\dfrac{n}{2} + 1} \right) \times \dfrac{2}{n}$
$Y = \dfrac{2}{{\not \prime }} \times \dfrac{{\not t}}{{{\gamma ^\prime }}} + 1 \times \dfrac{2}{n}$
Hence, $Y = \left( {1 + \dfrac{2}{n}} \right)$
3: Two perfect gases at absolute temperature ${{\text{T}}_1}$ and ${{\text{T}}_2}$ are mixed. There is no loss of energy. Find the temperature of the mixture if the masses of molecules are ${{\text{m}}_1}$ and ${{\text{m}}_2}$and number of molecules is ${{\text{n}}_1}$ and ${{\text{n}}_2}?$
Ans: There is no reciprocal interaction between the molecules in a perfect gas. Now, K.E of gas $ = \dfrac{1}{2}m{v^2}$
Energy equivalence is achieved by energy equivalence :
$\dfrac{1}{2}m{v^2} = \dfrac{3}{2}KT$
K.E of one gas $ = {n_1} \times \left( {\dfrac{3}{2}K{T_1}} \right) \to 1$ )
K.E. of other gas $ = {n_2} \times \left( {\dfrac{3}{2}K{T_2}} \right) \to 2$ )
Now , ${{\text{n}}_1},{{\text{n}}_2} = $ Number of molecules in gases
${\text{K}} = $ Bolt zman' Constant
${{\text{T}}_1},\;{{\text{T}}_2} \to $ Temperatures.
Total K.E. $ = \dfrac{3}{2}K\left( {{n_1}{T_1} + {n_2}{T_2}} \right)$ (adding equation 1)\&2)
Let we know that, ${\text{T}}$ be the absolute temperature of the mixture of gases
Then,
Total Kinetic energy $ = {n_1} \times \left( {\dfrac{3}{2}KT} \right) + {n_2} \times \left( {\dfrac{3}{2}KT} \right)$
Total K.E $ = \dfrac{3}{2}KT\left( {{n_1} + {n_2}} \right) \to 4$ )
Since there is no loss of energy, hence on equating e$^43$ ) & 4) for total K.E.: $ \to $
$\dfrac{{\not 3}}{{\not 2}}\not KT({n_1} + {n_2}) = \dfrac{{\not 3}}{{\not 2}}\not K\left( {{n_1}{T_1} + {n_2}{T_2}} \right)$
$T\left( {{n_1} + {n_2}} \right) = \left( {{n_1}{T_1} + {n_2}{T_2}} \right)$
Therefore,$T = \dfrac{{{n_1}{T_1} + {n_2}{T_2}}}{{{n_1} + {n_2}}}$
4. Derive Avogadro's law?
Ans: Avogadro's law asserts that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules. Consider two gases with similar volumes at the same temperature T and pressure${{\text{P}}^\prime }$.
Let ${{\text{M}}_1}$ =Mass of first gas
${{\text{M}}_2} = $ Mass of second gas
${{\text{C}}_1} = {{\text{C}}_2} = {\text{r}}.{\text{m}}.{\text{s}}$ velocity of gas molecules of 2 gases ${\text{m}}1/{\text{m}}2 = $ Mass of each molecule of gas
${{\text{M}}_1},\;{{\text{m}}_2} = $ Number of molecules of gas
Now, ${{\text{M}}_1} = {{\text{m}}_1}{{\text{n}}_1}$ and ${{\text{M}}_2} = {{\text{m}}_2}{{\text{n}}_2}$
From kinetic theory of gas: -
For first gas $ \Rightarrow P = \dfrac{{1{M_1}}}{{3V}}C_1^2 \to (1)$
For second gas $ \Rightarrow P = \dfrac{{1{M_2}}}{{3V}}C_2^2 \to (2)$
Now, Equating equation 1) 82) for pressure
$\dfrac{{1{M_1}}}{{3V}}C_1^2 = \dfrac{{1{M_2}}}{{3V}}C_2^2$
$\left. {{{\text{M}}_1}{\text{c}}_1^2 = {\text{M}}_2^2{\text{C}}_2^2 \to 3} \right)$
$\dfrac{{{\text{ AverageK}}{\text{.E }}}}{{{\text{ Molecule of first gas }}}} = \dfrac{{{\text{ Average KE}}}}{{{\text{ Moleculeof sec ond gas }}}} \Rightarrow $ for same temperatures
$\dfrac{1}{2}{M_1}C_1^2 = \dfrac{1}{2}{M_2}C_2^2$
$\left. {{{\text{M}}_1}{\text{C}}_1^2 = {{\text{M}}_2}{{\text{C}}_2}^2 \to 4} \right)$
Let ${\text{Cl}},{\text{C}}2 - {\text{Cn}} = $ Random velocities of gases molecules
Let Radom rectangular co-ordinates of $\eta $ - molecules
$x_1^2 + y_1^2 + z_1^2 = c_1^2$
So, ${x_n}^2 + {y_n}^2 + {z_n}^2 = {c_n}^2$
we have,
Initial Molomentum of ${\text{A}}, = {\text{m}}{{\text{x}}_1}$
on collision with wall, Momentum = -mx,
Change in Momentum $ = - {\text{m}}{{\text{x}}_1} - {\text{m}}{{\text{x}}_1}$
$ = - 2\;{\text{m}}{x_1}$
Let , The molecule in between the collisions of two walls OPKT and QRSL covers a distance $ = 2{\text{a}}$
So, time between 2 collisions $ = \dfrac{{2a}}{{{x_1}}}$
As Mornentum transferred in 1 second $ = 2\;{\text{m}}{x_1}{\text{x}}\dfrac{{{x_1}}}{{2a}} = \dfrac{{mx_{\text{g}}^2}}{a}$
From Newton's second law $ \Rightarrow {f_1} = \dfrac{{mx_1^2}}{a}$
$fn = \dfrac{{mx_n^2}}{a}$
Total force in ${\text{X}}$ -direction $ = {{\text{f}}_1} + {{\text{f}}_2} + \ldots {\text{fn}}$
$ = \dfrac{{mx_1^2}}{a} + \dfrac{{mx_2^2}}{a} + - - - - \dfrac{{mx_h^2}}{a}$
Pressure exerted on wall QRSL
$ = \dfrac{{Fx}}{{{a^2}}} = \dfrac{m}{{{a^2}}}\left( {x_1^2 + x_2^2 + - - - - x_n^2} \right)$
Dividing equation 4 ) by 3 )
$\dfrac{{{M_1}C_1^2}}{{{m_1}c_1^2}} = \dfrac{{{M_2}C_2^2}}{{{m_2}c_2^2}}\quad M = m \times n$
$\dfrac{{{m_1}{n_1}}}{{{m_1}}} = \dfrac{{{m_2}{n_2}}}{{{m_2}}}$
$ \Rightarrow $ I.e. Avogadro's law
${{\text{n}}_1} = {{\text{n}}_2}$
5. What are the assumptions of kinetic theory of gas?
Ans: The following are the assumptions of the kinetic theory of gases:
1) A gas is made up of a vast number of molecules that should all be elastic spheres and identical.
2) A gas's molecules are in a constant state of rapid and unpredictable mobility.
3) Gas molecules are extremely small in comparison to the distance between them.
4) There is no attraction or repulsio between the molecules.
5) Molecule collisions with one another and with the vessel's walls are perfectly elastic.
6. Estimate the fraction of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be $3{{A}^{0} }$.
Ans: Given, Diameter of an oxygen molecule, $d = 3A$
Radius, $r = \dfrac{d}{2} = \dfrac{3}{2} = 1.5{\quad _A} = 1.5 \times {10^{ - 8}}\;{\text{cm}}$
We know that the Actual volume occupied by 1 mole of oxygen gas at $ TP = 22400\;{\text{c}}{{\text{m}}^3}$
Let Molecular volume of oxygen gas, $V = \dfrac{4}{3}\pi {r^3} - N$
Where, $N$ is Avogadro's number $ = 6.023 \times 1023$ molecules/mole
$\therefore V = \dfrac{4}{3}3.14 \times {\left( {1.5 \times {{10}^{ - 8}}} \right)^3} \times 6.023 \times {10^{23}} = 8.51\;{\text{c}}{{\text{m}}^3}$
Hence, the Ratio of the molecular volume to the actual volume of oxygen $ = \dfrac{{8.51}}{{22400}}$
$ = 3.8 \times {10^{ - 4}}$
7. Estimate the total number of air molecules (inclusive of oxygen, nitrogen, water vapour and other constituents) in a room of capacity $25.0\;{{\text{m}}^3}$ at a temperature of ${{27}^{0} }{\text{C}}$.
Ans: Given is, Volume of the room, $V = 25.0\;{{\text{m}}^3}$
Temperature of the room, $T = {{27}^{0}}{\text{C}} = 300\;{\text{K}}$
Pressure in the room, $P = 1\;{\text{atm}} = 1 \times 1.013 \times {10^5}\;{\text{Pa}}$
We get, The ideal gas equation relating pressure $(P)$, Volume (V), and absolute temperature ( $T$ ) can be written as:
$PV = {k_B}NT$
Where,
${K_B}$ is Boltzmann constant $ = 1.38 \times {10^{ - 23}}\;{{\text{m}}^2}\;{\text{kg}}\;{{\text{s}}^{ - 2}}\;{{\text{K}}^{ - 1}}$
$N$ is the number of air molecules in the room
Now,
$\therefore N = \dfrac{{PV}}{{{k_5}T}}$
$ = \dfrac{{1.013 \times {{10}^5} \times 25}}{{1.38 \times {{10}^{ - 25}} \times 300}}$
$ = 6.11 \times {10^{26}}$ molecules
As a result, the total number of air molecules in a room is $6.11 \times {10^{26}}$.
8. From a certain apparatus, the diffusion rate of hydrogen has an average value of $28.7$ ${\text{cm}}3\;{{\text{s}}^{ - 1}}$. The diffusion of another gas under the same conditions is measured to have an average rate of $7.2\;{\text{c}}{{\text{m}}^3}\;{{\text{s}}^{ - 1}}$. Identify the gas.(Hint: Use Graham's law of diffusion: ${{\text{R}}_1}/{{\text{R}}_2} = {\left( {{{\mathbf{M}}_2}/{{\mathbf{M}}_1}} \right)^{1/2}}$, where ${{\mathbf{R}}_1},{{\mathbf{R}}_2}$ are)
diffusion rates of gases 1 and 2 , and ${{\mathbf{M}}_1}$ and ${{\mathbf{M}}_2}$ their respective molecular masses. The law is a simple consequence of kinetic theory.)
Ans: Given,
Rate of diffusion of hydrogen, ${R_1} = 28.7\;{\text{c}}{{\text{m}}^3}\;{{\text{s}}^{ - 1}}$
Rate of diffusion of another gas, ${R_2} = 7.2\;{\text{c}}{{\text{m}}^3}\;{{\text{s}}^{ - 1}}$
According to Graham's Law of diffusion, we have:
$\dfrac{{{R_1}}}{{{R_2}}} = \sqrt {\dfrac{{{{\mathbf{M}}_2}}}{{{{\mathbf{M}}_1}}}} $
Where,
${M_1}$ is the molecular mass of hydrogen $ = 2.020\;{\text{g}}$
${M_2}$ is the molecular mass of the unknown gas
Now putting value in given,
$\therefore {{\mathbf{M}}_2} = {{\mathbf{M}}_1}{\left( {\dfrac{{{R_1}}}{{{R_2}}}} \right)^2}$
$ = 2.02{\left( {\dfrac{{28.7}}{{7.2}}} \right)^2} = 32.09\;{\text{g}}$
32 g is the oxygen molecular mass. As a result, oxygen is the unknown gas.
4 Marks Questions
1. Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP: 1 atmospheric pressure $0{}^\circ \text{C}$. Show that it is $22.4$ liters.
Ans: The relationship between pressure (P), volume (V), and absolute temperature (T) is known as the ideal gas equation.
$PV = $ net
Where,
$R$ is the universal gas constant $ = 8.314\;{\text{J}}\;{\text{mo}}{{\text{l}}^{ - 1}}\;{{\text{K}}^{ - 1}}$
$n = $ Number of moles $ = 1$
$T = $ Standard temperature $ = 273\;{\text{K}}$
$P = $ Standard pressure $ = 1\;{\text{atm}} = 1.013 \times {10^5}{\text{N}}{{\text{m}}^{ - 2}}$
Formula is,
$\therefore V = \dfrac{{nRT}}{P}$
Now put the value,
$ = \dfrac{{1 \times 8.314 \times 273}}{{1.013 \times {{10}^5}}}$
$ = 0.0224\;{{\text{m}}^3}$
i.e V$ = 22.4$ liters
As a result, a gas's molar volume at STP is 22.4 litres.
2. Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic), and the third contains uranium hexafluoride (polyatomic). Do the vessels contain equal number of respective molecules? Is the root mean square speed of molecules the same in the three cases? If not, in which case is the largest?
Ans: Yes. All of them have the same number of molecules in them.
No. Neon has the highest root mean square speed.
The three jars have the same volume because their capacities are equal.
As a result, the pressure, volume, and temperature of each gas are the same.
The three vessels will each contain an equal quantity of the corresponding molecules, according to Avogadro's law. This is the same as Avogadro's number.
$N = 6.023 \times {10^{23}}$.
The root mean square speed $\left( {{v_{{\text{ru}}}}} \right)$ of a gas of mass ${\text{m}}$, and temperature $T$, is given relation is :
${v_{r = }} = \sqrt {\dfrac{{3kT}}{m}} $
Here, $k$ is Boltzmann constant
As we know the given gases, $k$ and $T$ are constants.
Hence ${v_{{r_{14}}}}$ depends only on the mass of the atoms, i.e.,
${v_{rne}} \propto \sqrt {\dfrac{1}{m}} $
As a result, the root mean square speed of the molecules in each of the three scenarios differs. The mass of neon is the smallest of the three elements: neon, chlorine, and uranium hexafluoride. As a result, among the supplied gases, neon has the fastest root mean square speed
3. A metre-long narrow bore held horizontally (and closed at one end) contains a $76\;{\text{cm}}$long mercury thread, which traps a $15\;{\text{cm}}$ column of air. What happens if the tube is held vertically with the open end at the bottom?
Ans: Given By the Length of the narrow bore, $L = 1\;{\text{m}} = 100\;{\text{cm}}$
Length of the mercury thread, $l = 76\;{\text{cm}}$
Length of the air column between mercury and the closed end, ${l_a} = 15\;{\text{cm}}$
Now,The mercury length that occupies the air space is because the bore is kept vertically in air with the open end at the bottom : $100 - (76 + 15) = 9\;{\text{cm}}$
Hence, the total length of the air column $ = 15 + 9 = 24\;{\text{cm}}$
Let $h\;{\text{cm}}$ of mercury flow out as a result of atmospheric pressure.
- Length of the air column in the bore $ = 24 + h\;{\text{cm}}$
And, length of the mercury column $ = 76 - h\;{\text{cm}}$
Initial pressure, ${P_1} = 76\;{\text{cm}}$ of mercury
Initial volume, ${V_1} = 15\;{\text{c}}{{\text{m}}^3}$
Final pressure, ${P_2} = 76 - (76 - h) = h\;{\text{cm}}$ of mercury
Final volume, ${V_2} = (24 + h){\text{c}}{{\text{m}}^3}$
Temperature remains constant throughout the process.
$\therefore {P_1}{V_1} = {P_2}{V_2}$
$76 \times 15 = h(24 + h)$
${h_2} + 24h - 1140 = 0$
$\therefore h = \dfrac{{ - 24 \pm \sqrt {{{(24)}^2} + 4 \times 1 \times 1140} }}{{2 \times 1}}$
$ = 23.8\;{\text{cm}}$ or $ - 47.8\;{\text{cm}}$
Height can't possibly be a disadvantage. As a result, 23.8 cm of mercury will flow out of the hole, leaving 52.2 in the chamber. The length of the air column will be. $24 + 23.8 = 47.8\;{\text{cm}}$.
4. An air bubble of volume $1.0\;{\text{cm}}3$ rises from the bottom of a lake $40\;{\text{m}}$ deep at a temperature of ${{35}^{0}}{\text{C}}$ ?
Ans: Given is, Volume of the air bubble, ${V_1} = 1.0\;{\text{c}}{{\text{m}}^3} = 1.0 \times {10^{ - 6}}\;{{\text{m}}^3}$
Bubble rises to height, $d = 40\;{\text{m}}$
Temperature at a depth of $40\ \text{m},{{T}_{1}}=12{}^\circ \text{C}=285\ \text{K}$
Temperature at the surface of the lake, ${{T}_{2}}=35{}^\circ \text{C}=308\ \text{K}$
The pressure on the surface of the lake:
${P_2} = 1\;{\text{atm}} = 1 \times 1.013 \times {10^5}\;{\text{Pa}}$
The pressure at the depth of $40\;{\text{m}}$ :
${P_1} = 1\;{\text{atm}} + d\rho {\text{g}}$
Given ,
$\rho $ is the density of water $ = {10^3}\;{\text{kg}}/{{\text{m}}^3}$
${\text{g}}$ is the acceleration due to gravity $ = 9.8\;{\text{m}}/{{\text{s}}^2}$
$\therefore {P_1} = 1.013 \times {10^5} + 40 \times {10^3} \times 9.8$
$ = 493300\;{\text{Pa}}$
We have: $\dfrac{{{P_1}{V_1}}}{{{T_1}}} = \dfrac{{{P_2}{V_2}}}{{{T_2}}}$
Where, ${V_2}$ is the volume of the air bubble when it reaches the surface
${V_2} = \dfrac{{P{V_1}{T_2}}}{{{T_1}{P_2}}}$
Now put the value,
$ = \dfrac{{(493300)\left( {1.0 \times {{10}^{ - 5}}} \right)308}}{{285 \times 1.013 \times {{10}^5}}}$
$ = 5.263 \times {10^{ - 6}}\;{{\text{m}}^3}$ or $5.263\;{\text{c}}{{\text{m}}^3}$
As a result, the volume of the air bubble decreases as it approaches the surface. $5.263\;{\text{c}}{{\text{m}}^3}$.
5. Estimate the average thermal energy of a helium atom at
Ans:
(i) room temperature $\left( 27{}^\circ \text{C} \right)$
At room temperature, calculate the average thermal energy of a helium atom. $T=27{}^\circ \text{C}=300\ \text{K}$
Now, Average thermal energy $= \dfrac{3}{2}kT$
Let, $k$ is Boltzmann constant $= 1.38 \times {10^{ - 23}}\;{{\text{m}}^2}\;{\text{kg}}\;{{\text{s}}^{ - 2}}\;{{\text{K}}^{ - 1}}$
$\therefore \dfrac{3}{2}kT = \dfrac{3}{2} \times 1.38 \times {10^{ - 98}} \times 300$
$ = 6.21 \times {10^{ - 21}}J$
As a result, at ambient temperature, the average thermal energy of a helium atom is $\left({{27}^{0} }{\text{C}} \right)$ is $6.21 \times {10^{ - 21}}J$
(ii) the temperature on the surface of the Sun (6000 K)
Determine the average thermal energy of a helium atom at the sun's surface. $T = 6000\;{\text{K}}$
Now, Average thermal energy $ = \dfrac{3}{2}kT$
$\dfrac{3}{2} \times 1.38 \times {10^{ - 38}} \times 6000$
$ = 1.241 \times {10^{ - 18}}J$
As a result, a helium atom on the sun's surface has an average thermal energy of $1.241 \times {10^{ - 19}}J$
(iii) the temperature of 10 million Kelvin (the typical core temperature in the case of a star).
Find the average thermal energy of a helium atom At temperature, $T = 107\;{\text{K}}$
Now, Average thermal energy $ = \dfrac{3}{2}kT$
$\dfrac{3}{2} \times 1.38 \times {10^{ - 38}} \times {10^7}$
$ = 2.07 \times {10^{ - 16}}J$
As a result, the average thermal energy of a helium atom at a star's core is $2.07 \times {10^{ - 16}}J$.
6. At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the rms speed of a helium gas atom at $ - {{20}^{0}}{\text{C}}?$ (atomic mass of ${\text{Ar}} = $ $39.9{\text{u}}$, of ${\text{He}} = 4.0{\text{u}}$ ).
Ans: Given data is,
Temperature of the helium atom, ${T_F} = - {{20}^{0}}{\text{C}} = 253\;{\text{K}}$
Atomic mass of argon, ${M_A} = 39.9{\text{u}}$
Atomic mass of helium, ${M_{He}} = 4.0{\text{u}}$
Let, ${\left( {{v_{r = \infty }}} \right)_d}$ be the rms speed of argon.
Let ${\left( {{v_{rwe}}} \right)_{Ht}}$ be the rms speed of helium.
We have , The rms speed of argon is given by:
${\left( {{v_{n + \xi }}} \right)_A} = \sqrt {\dfrac{{3R{T_A}}}{{{M_{\Delta t}}}}} $
Where,
$R$ is the universal gas constant
${T_A}$ is temperature of argon gas
Now, The rms speed of helium is given by:
${\left( {{v_{rms}}} \right)_{Ht}} = \sqrt {\dfrac{{3R{T_R}}}{{{M_H}}}} \ldots $ (ii)
It is given that:
${\left( {{v_{rre}}} \right)_{At}} = {\left( {{v_{rwb}}} \right)_B}$
$\sqrt {\dfrac{{3R{T_{AV}}}}{{{M_A}}}} = \sqrt {\dfrac{{3R{T_B}}}{{{M_H}}}} $
$\dfrac{{{T_\Delta }}}{{{M_\Delta }}} = \dfrac{{{T_B}}}{{{M_{Ht}}}}$
${T_A} = \dfrac{{{T_{{H_t}}}}}{{{M_{{H_t}}}}} \times {M_\Delta }$
$ = \dfrac{{253}}{4} \times 39.9$
$ = 2523.675 = 2.52 \times {10^3}\;{\text{K}}$
As a result, the argon atom's temperature is $2.52 \times {10^3}\;{\text{K}}$.
5 Marks Questions
1. Derive an expression for the pressure due to an ideal gas?
Ans: Consider an ideal gas confined in an OPRSTKL cubical container with capacity on each side a. ${\text{V}}$ now, ${\text{V}} = {{\text{a}}^3}$ ((Side) $^3 = $ volume of cube)
Where, ${\text{n}} = $ Molecule of gas
${\text{m}} = $ Mass of each molecule
${\text{M}} = {\text{mxn}} = $ Mass of gas
Now Similarly, ${\text{Py}} = \dfrac{m}{{{a^3}}}\left( {y_1^2 + {y_2}^2 + - - {y_n}^2} \right)$
$Pz = \dfrac{m}{{{a^3}}}\left( {z_1^2 + {z_2}^2 + - - {z_n}^2} \right)$
${\text{P}} = $ Total pressure $ = \dfrac{{{P_x} + {P_y} + {P_z}}}{3}$
Put the following equation as per formula,
$ = \dfrac{1}{3}\left[ {\dfrac{m}{{{a^2}}}\left( {x_1^2 + x_2^2 + - - x_n^2} \right) + \dfrac{m}{{{a^2}}}\left( {y_1^2 + y_2^2 + - - y_n^2} \right) + \dfrac{m}{{{a^3}}}\left( {z_1^2 + z_2^2 + - - z_n^2} \right)} \right]$
${\text{P}} = \dfrac{m}{{3{a^3}}}\left[ {\left( {x_1^2 + y_1^2 + {z_1}^2} \right) + - - - \left( {{x_n}^2 + {y_n}^2 + {z_n}^2} \right)} \right](\because $ from equation A)
$P = \dfrac{m}{{3v}}\left[ {C_1^2 + C_2^2 + - - C_n^2} \right]$
$ \to $ Multiply & divide by ${\text{n}}$ (no of molecules of gas)
$P = \dfrac{{1mpn}}{{3v}}\left[ {\dfrac{{C_1^2 + C_2^2 + - - {C_n}^2}}{n}} \right]$
${\text{P}} = \dfrac{{1{\text{M}}}}{{3v}}{C^2}$
${c^2} = \dfrac{{C_1^2 + C_2^2 + - - C_n^2}}{n}$ or $C = \sqrt {\dfrac{{C_1^2 + C_2^2 + - - C_n^2}}{n}} $
${\text{C}} = {\text{r}}.{\text{ms}}$. velocity of gas.
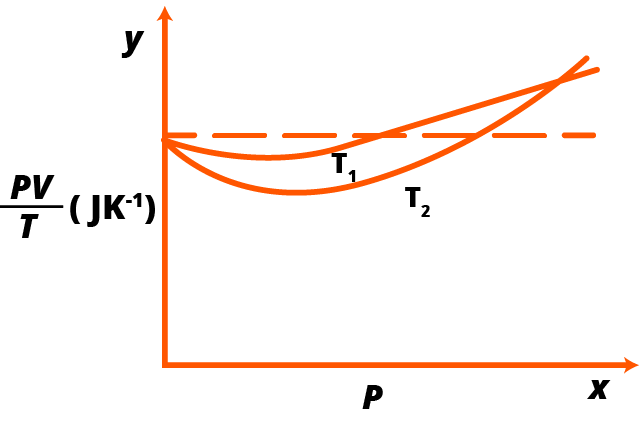
2: Figure 13.8 shows plot of $PV\mid T$ versus Poor $1.00 \times {10^{ - 3}}\;{\text{kg}}$ of oxygen gas at two different temperatures.
Molecular mass of ${H_2} = 2.02u,\,of\,O2 = 32.0{\text{u}},R = 8.31\;{\text{Jmo}}{1^{ - 1}}\;{{\text{K}}^{ - 1}}$
Ans:
(a) What does the dotted plot signify?
The dotted plot in the graph, in which the ratio is equal, depicts the ideal behaviour of the gas Is a constant quality (where is the number of moles and is the universal gas constant). It isn't affected by the gas's pressure.
(b) Which is true: ${T_1} > {T_2}$ or ${T_1} < {T_2}?$
The dotted plot in the graph represents the ideal gas. The gas's temperature curve is closer to the dotted plot than the gas's temperature curve. When a real gas's temperature rises, it begins to behave like an ideal gas. As a result, is true for the given plot.
(c) What is the value of $PV/T$ where the curves meet on the $y$ -axis?
The value of the ratio $PV/T$, where the two curves meet, is ${\mu _R}$. This is because the ideal gas equation is given as:
$PV = \mu RT$
$\dfrac{{PV}}{T} = \mu R$
Where,
$P$ is the pressure
$T$ is the temperature
$V$ is the volume
$\mu $ is the number of moles
$R$ is the universal constant
Molecular mass of oxygen $ = 32.0\;{\text{g}}$
Mass of oxygen $ = 1 \times {10^{ - 3}}\;{\text{kg}} = 1\;{\text{g}}$
We have, $R = 8.314\;{\text{J}}\;{\text{mol}}{{\text{e}}^{ - 1}}\;{{\text{K}}^{ - 1}}$
$\therefore \dfrac{{PV}}{T} = \dfrac{1}{{32}} \times 8.314$
$ = 0.26\;{\text{J}}{K^{ - 1}}$
Therefore, the value of the ratio $PV/T$, where the curves meet on the $y$ -axis, is
$0.26\;{\text{J}}{K^{ - 1}}$
(d) If we obtained similar plots for $1.00 \times {10^{ - 3}}\;{\text{kg}}$ of hydrogen, would we get the same value of $PV/T$ at the point where the curves meet on the $y$ -axis? If not, what mass of hydrogen yields the same value of $PV/T$ (for low pressure high temperature region of the plot)?
If we obtain similar plots for $1.00 \times {10^{ - 3}}\;{\text{kg}}$ of hydrogen, then we will not get the same value of $PV/T$ at the point where the curves meet the $y$ -axis. This is because the molecular mass of hydrogen ( $(2.02{\text{u}})$ is different from that of oxygen (32.0 u).
We have:
$\dfrac{{PV}}{T} = 0.26J{K^{ - 1}}$
Now ,
$R = 8.314\;{\text{J}}\;{\text{mol}}{e^{ - 1}}\;{{\text{K}}^{ - 1}}$
Molecular mass $(M)$ of ${H_2} = 2.02{\text{u}}$
Let $\dfrac{{PV}}{T} = \mu R$ at constant temperature
where, $\mu = \dfrac{m}{M}$
$m$ - Mass of ${H_2}$
$\therefore m = \dfrac{{PV}}{T} \times \dfrac{M}{R}$
$ = \dfrac{{0.26 \times 2.02}}{{8.31}}$
$ = 6.3 \times {10^{ - 2}}g = 6.3 \times {10^{ - 5}}\;{\text{kg}}$
Therefore,, $6.3 \times {10^{ - 5}}\;{\text{kg}}$ of ${H_2}$ will yield the same value of $PV/T$.
3: An oxygen cylinder of volume 30 litres has an initial gauge pressure of 15 atm and a temperature of ${{27}^{0}}{\text{C}}$. After some oxygen is withdrawn from the cylinder, the gauge pressure drops to 11 atm and its temperature drops to ${{17}^{0}}{\text{C}}$. Estimate the mass of oxygen taken out of the cylinder $\left( {R = 8.31\;{\text{J}}\;{\text{mo}}{{\text{l}}^{ - 1}}\;{{\text{K}}^{ - 1}}} \right.$, molecular mass of ${{\text{O}}_2} = 32{\text{u}}$ ).
Ans: Given,
Volume of oxygen, ${V_1} = 30$ litres - $30 \times {10^{ - 3}}\;{{\text{m}}^3}$
Gauge pressure, ${P_1} - 15\;{\text{atm}} = 15 \times 1.013 \times {10^5}\;{\text{Pa}}$
Temperature, ${T_1} = {{27}^{0}}{\text{C}} = 300\;{\text{K}}$
Universal gas constant, $R = 8.314\;{\text{J}}\;{\text{mo}}{{\text{l}}^{ - 1}}{{\mathbf{K}}^{ - 1}}$
Let the initial number of moles of oxygen gas in the cylinder be ${n_1}$.
Now we calculate the given equation,
${P_1}{V_1} = {n_1}R{T_1}$
$\therefore {n_1} = \dfrac{{{P_1}{V_1}}}{{R{T_1}}}$
$ = \dfrac{{15.195 \times {{10}^5} \times 30 \times {{10}^{ - 3}}}}{{(8.314) \times 300}} = 18.276$
But, ${n_1} = \dfrac{{{m_1}}}{M}$
Where,
${m_1} = $ Initial mass of oxygen
$M$ - Molecular mass of oxygen $ = 32\;{\text{g}}$
$\therefore {m_1} = {n_1}M = 18.276 \times 32 = 584.84\;{\text{g}}$
The pressure and temperature drop after some oxygen is removed from the cylinder.
Given is , Volume, ${V_2} = 30$ litres $ = 30 \times {10^{ - 3}}\;{{\text{m}}^3}$
Gauge pressure, ${P_2} = 11\;{\text{atm}} = 11 \times 1.013 \times {10^5}\;{\text{Pa}}$
Temperature, ${T_2} = {{17}^{0}}{\text{C}} = 290\;{\text{K}}$
Let ${n_2}$ be the number of moles of oxygen left in the cylinder.
The gas equation is given as:
${P_2}{V_2} = {n_2}R{T_2}$
$\therefore {n_2} = \dfrac{{{P_2}{V_2}}}{{R{T_2}}}$
$ = \dfrac{{11.143 \times {{10}^5} \times 30 \times {{10}^{ - 3}}}}{{8.314 \times 290}} = 13.86$
But, ${n_2} = \dfrac{{{m_2}}}{M}$
Where,
${m_2}$ is the mass of oxygen remaining in the cylinder
$\therefore {m_2} = {n_2}M = 13.86 \times 32 = 453.1\;{\text{g}}$
The mass of oxygen taken out of the cylinder can be calculated using the following formula:
Mass of oxygen in the cylinder at the start - Mass of oxygen in the cylinder at the end$ = {m_1} - {m_2}$
$ = 584.84\;{\text{g}} - 453.1\;{\text{g}}$
$ = 131.74\;{\text{g}}$
$ = 0.131\;{\text{kg}}$
Hence, $0.131\;{\text{kg}}$ of oxygen is taken out of the cylinder.
4. Estimate the mean free path and collision frequency of a nitrogen molecule in a cylinder containing nitrogen at $2.0\;{\text{atm}}$ and temperature ${{17}^{0}}{\text{C}}$. Take the radius of a nitrogen molecule to be roughly $1.0_A^o$. Compare the collision time with the time the molecule moves freely between two successive collisions (Molecular mass of $\left. {{{\text{N}}_2} = 28.0{\text{u}}} \right)$
Ans: We know that the , Mean free path $ = 1.11 \times {10^{ - 7}}\;{\text{m}}$
Collision frequency $ = 4.58 \times {10^9}\;{{\text{s}}^{ - 1}}$
Successive collision time $ \approx 500 \times $ (Collision time)
Pressure inside the cylinder containing nitrogen, $P = 2.0\;{\text{atm}} = 2.026 \times {10^5}\;{\text{Pa}}$
Let , Temperature inside the cylinder, $T = {}^ \circ {A^{{{17}^{0} }{\text{C}} - 290\;{\text{K}}}}$
Radius of a nitrogen molecule, $r = 1.0 - 1 \times {10^{10}}\;{\text{m}}$
Diameter, $d = 2 \times 1 \times {10^{10}} = 2 \times {10^{10}}\;{\text{m}}$
Molecular mass of nitrogen, $M = 28.0\;{\text{g}} = 28 \times {10^{ - 3}}\;{\text{kg}}$
The relationship gives the nitrogen root mean square speed :
${v_{n{\text{se}}}} = \sqrt {\dfrac{{3RT}}{M}} $
Where,
$R$ is the universal gas constant $ = 8.314\;{\text{J}}\;{\text{mol}}{{\text{e}}^{ - 1}}{K^{ - 1}}$
$\therefore {v_{me}} = \sqrt {\dfrac{{3 \times 8.314 \times 290}}{{28 \times {{10}^{ - 4}}}}} = 508.26\;{\text{m}}/{\text{s}}$
The mean free path ( ${\text{b}}$ is given by the relation:
Now ,$l = \dfrac{{kT}}{{\sqrt 2 \times {d^2} \times P}}$
Where,
$K$ is the Boltzmann constant $ = 1.38 \times {10^{ - 21}}\;{\text{kg}}\;{{\text{m}}^2}\;{{\text{s}}^{ - 2}}\;{{\text{K}}^{ - 1}}$
$\therefore l = \dfrac{{1.38 \times {{10}^{ - 2}} \times 290}}{{\sqrt 2 \times 3.14 \times {{\left( {2 \times {{10}^{ - 12}}} \right)}^2} \times 2.026 \times {{10}^5}}}$
$ = 1.11 \times {10^{ - 7}}m$
Collision frequency $ = \dfrac{{{v_{rec}}}}{l}$
$ = \dfrac{{508.26}}{{1.11 \times {{10}^{ - 7}}}} = 4.58 \times 109{s^{ - 1}}$
Collision time is given as:
$T = \dfrac{d}{{{v_{nsL}}}}$
$ = \dfrac{{2 \times {{10}^{ - 10}}}}{{508.26}} = 3.93 \times {10^{ - 13}}s$
The amount of time that passes between subsequent collisions :
${T^\prime } = \dfrac{l}{{{v_{rm}}}}$
$ = \dfrac{{1.11 \times {{10}^{ - 7}}}}{{508.26\;{\text{m}}/{\text{s}}}} = 2.18 \times {10^{ - 10}}s$
$\therefore \dfrac{{{T^\prime }}}{T} = \dfrac{{2.18 \times {{10}^{ - 10}}}}{{3.93 \times {{10}^{ - 11}}}} = 500$
As a result, the period between subsequent collisions is 500 times that of a single collision.
5. A gas in equilibrium has uniform density and pressure throughout its volume. This is strictly true only if there are no external influences. A gas column under gravity, for example, does not have uniform density (and pressure). As you might expect, its density decreases with height. The precise dependence is given by the so-called law of atmospheres
${n_2} = {n_1}\exp \left[ { - mg\left( {{h_2} - {h_1}} \right)/{k_{\text{B}}}T} \right]$
Where $nl,{n_1}$ refer to number density at heights ${h_2}$ and ${h_1}$ respectively. Use this relation to derive the equation for sedimentation equilibrium of a suspension in a liquid
Column:
${n_2} = {n_1}\exp \left[ { - mg{N_A}\left( {\rho - {P^\prime }} \right)\left( {{h_2} - {h_1}} \right)/(\rho RT)} \right]$
Where $\rho $ is the density of the suspended particle, and ${\rho ^\prime }$ that of surrounding medium . I ${N_A}$ is Avogadro's number, and $R$ the universal gas constant.] [Hint: use Archimedes principle to find the apparent
Ans: We have, according to the rule of atmospheres,
${n_2} = {n_1}\exp \left[ { - mg\left( {{h_2} - {h_1}} \right)/{k_B}T} \right] \ldots (i)$
Where,
${n_1}$ is the number density at height ${h_1},and{n_2}$ is the number density at height ${h_2}$
${m_g}$ is the weight of the particle suspended in the gas column
Density of the medium $ = {\{{rho}^{0} }$
Density of the suspended particle $ = \rho $
Mass of one suspended particle $ = {{\mathbf{m}}^\prime }$
Mass of the medium displaced $ = m$
Volume of a suspended particle = $V$
The effective weight of a suspended particle in a liquid column is given by Archimedes' principle for a particle suspended in a liquid column as :
Let , Weight of the medium displaced - Weight of the suspended particle
$ = mg - {m^\prime }g$
$ = mg - V{\rho ^\prime }g = mg - \left( {\dfrac{m}{\rho }{\rho ^\prime }g} \right)$
$mg\left( {1 - \dfrac{{{\rho ^\prime }}}{\rho }} \right) - \ldots \ldots .(1i)$
Now Gas constant, ${\text{R}} = {k_B}\;{\text{N}}$
${k_B} = \dfrac{R}{N} \ldots (\omega i)$
Substituting equation (ii) in place of $m g$ in equation $(i)$ and then using equation (iii), we get:
${n_2} = {n_1}\exp \left[ { - mg\left( {{h_2} - {h_1}} \right)/{k_B}T} \right]$
$ - {n_1}\exp \left[ { - mg\left( {1 - \dfrac{{{\rho ^\prime }}}{\rho }} \right)\left( {{h_2} - {h_1}} \right)\dfrac{N}{{RT}}} \right]$
$ = {n_2}\exp \left[ { - mg\left( {\rho - {\rho ^\prime }} \right)\left( {{h_2} - {h_1}} \right)\dfrac{N}{{RT\rho }}} \right]$
6. Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms:
Substance | Atomic Mass (u) | Density $\left( {{{10}^3}{\mathbf{Kg}}_{\mathbf{m}}^{ - 3}} \right)$ |
Carbon (diamond) Gold Nitrogen (liquid) Lithium Fluorine (liquid) | 12.01 197.00 14.01 6.94 19.00 | 2.22 19.32 1.00 0.53 1.14 |
(Hint: Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro's number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few ${}^ \circ {A^{1.}}$.)
Ans: Determine the atomic sizes in the range of a few Radius ${}^ \circ {A^{1.}}$.
Substance | Radius (${}^ \circ {A^{1.}}$) |
Carbon (diamond) Gold Nitrogen (liquid) Lithium Fluorine (liquid) | 1.29 1.59 1.77 1.73 1.88 |
Atomic mass of a substance $ = M$
Density of the substance $ = \rho $
Avogadro's number $ = {\text{N}} = 6.023 \times {10^2}$
Volume of each atom $ = \dfrac{4}{3}\pi {r^3}$
Volume of $N$ number of molecules $ = \dfrac{4}{3}\pi {r^3}N \ldots (i)$
volume of one mole of a substance $ = \dfrac{M}{\rho } \ldots (ii)$
$ = \dfrac{4}{3}\pi {r^3}$
$N = \dfrac{M}{\rho }$
$\therefore r = \sqrt[3]{{\dfrac{{3.M}}{{4\pi \rho N}}}}$
For carbon:
$M = 12.01 \times {10^{ - 3}}\;{\text{kg}}$
$\rho = 2.22 \times {10^3}\;{\text{kg}}\;{{\text{m}}^{ - 3}}$
$\therefore r = {\left( {\dfrac{{3 \times 12.1 \times {{10}^{ - 4}}}}{{4\pi \times 2.22 \times {{10}^9} \times 6.023 \times {{10}^2}}}} \right)^{\dfrac{1}{3}}} = 1.29\dot A$
As a result, a carbon atom's radius is 1.29 A.
For the sake of gold:
$M - 197.00 \times {10^{ - 1}}\;{\text{kg}}$
$\rho = 19.32 \times {10^3}\;{\text{kg}}\;{{\text{m}}^{ - 3}}$
$\therefore r = {\left( {\dfrac{{3 \times 197 \times {{10}^{ - 1}}}}{{4\pi \times 19.32 \times {{10}^3} \times 6.023 \times {{10}^2}}}} \right)^{\dfrac{1}{3}}} = 1.59{}^ \circ A$
As a result, a gold atom's radius is 1.59.
In the case of liquid nitrogen:
$M = 14.01 \times {10^{ - 1}}\;{\text{kg}}$
$\rho = 1.00 \times {10^3}\;{\text{kg}}\;{{\text{m}}^{ - 1}}$
$\therefore r = {\left( {\dfrac{{3 \times 14.01 \times {{10}^{ - 1}}}}{{4\pi \times 1.00 \times {{10}^3} \times 6.23 \times {{10}^{21}}}}} \right)^{\dfrac{1}{3}}} = {{1.77}^{0} }A$
As a result, a liquid nitrogen atom's radius is 1.77 ${A}^{0}$ .
In the case of lithium:
$M = 6.94 \times {10^{ - 1}}\;{\text{kg}}$
${\text{p}} = 0.53 \times {10^{\text{i}}}{\text{kg}}{{\text{m}}^{ - 3}}$
$\therefore r = {\left( {\dfrac{{3 \times 6.94 \times {{10}^{ - 3}}}}{{4\pi \times 0.53 \times {{10}^1} \times 6.23 \times {{10}^{41}}}}} \right)^{\dfrac{1}{3}}} - 1.73{}^ \circ A$
As a result, the radius of a lithium atom is – 1.73 ${A}^{0}$ For liquid fluorine, the radius of a lithium atom is :
$M = 19.00 \times {10^{ - 3}}\;{\text{kg}}$
$\rho = 1.14 \times {10^3}kg$
Related Study Materials for Class 11 Physics Chapter 12
S.No | Important Other Links for Class 11 Physics Chapter 12 |
1. | |
2. |
CBSE Class 11 Physics Chapter-wise Important Questions
CBSE Class 11 Physics Chapter-wise Important Questions and Answers cover topics from other chapters, helping students prepare thoroughly by focusing on key topics for easier revision.
S.No | Chapter-wise Important Questions for Class 11 Physics |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | Chapter 6 - Systems of Particles and Rotational Motion Questions |
7 | |
8 | |
9 | |
10 | |
11 | |
12 | |
13 |
Additional Study Materials for Class 11 Physics
S.No | Study Materials for Class 11 Physics |
1 | |
2 | |
3 | |
4 |
FAQs on CBSE Important Questions for Class 11 Physics Kinetic Theory - 2025-26
1. What are the most important questions students should focus on in Class 11 Physics Chapter 12 – Kinetic Theory as per CBSE 2025–26 exam trends?
- Five-mark questions: Derivation of the pressure of an ideal gas, justification of gas laws using kinetic theory, or explaining mean free path and its dependence on various parameters.
- Three-mark questions: Application-based numericals involving root mean square velocity, gas laws, or distinguishing real and ideal gas behavior.
- One-mark questions: Definitions (mean free path, ideal gas), basic laws (Avogadro's, Boyle's), and key postulates of kinetic theory. Focus on derivations, numerical applications, and concept-based HOTS (Higher Order Thinking Skills) as seen in recent CBSE papers.
2. What are the key postulates of kinetic theory of gases that are frequently asked in CBSE Class 11 exams?
- Gases consist of a large number of tiny, identical molecules in continuous rapid motion.
- The volume occupied by molecules is negligible compared to the total volume of the gas.
- There are no intermolecular forces between molecules except during collisions.
- Collisions between molecules and with container walls are perfectly elastic.
- The average kinetic energy of gas molecules is directly proportional to absolute temperature.
3. Why do real gases show deviation from ideal behavior as per kinetic theory, and under what conditions do they behave ideally?
Real gases deviate from ideal behavior at high pressure and low temperature because intermolecular forces and the finite volume of molecules become significant. However, at low pressure and high temperature, gas molecules are far apart and intermolecular interactions and volume effects become negligible, so real gases approach ideal gas behavior.
4. What is mean free path, and which factors does it depend upon in the context of kinetic theory?
Mean free path is the average distance a molecule travels between two successive collisions. It depends on atmospheric pressure (inversely), temperature (directly), and the diameter of the molecules (inversely related to square of the diameter as per CBSE syllabus).
5. Explain the law of equipartition of energy and its significance in kinetic theory for board exams.
The law of equipartition of energy states that each degree of freedom contributes an equal amount of energy, i.e., ½ kT per degree of freedom per molecule. In kinetic theory, this explains how total internal energy of a gas is partitioned among translational, rotational, and vibrational motion. It is crucial for explaining specific heats and forms a HOTS question topic.
6. How can the pressure of an ideal gas be derived from the kinetic theory model? (Frequently asked 5-mark derivation)
- Assume a gas with 'N' molecules, each of mass 'm', in a container of volume 'V'.
- From kinetic considerations, derive force due to molecule's collisions on container wall.
- Sum contributions for all directions and molecules to show P = (1/3) × (N/V) × m × c2, where c2 is average squared speed.
- Conclude that pressure is proportional to average kinetic energy density, a standard derivation as per CBSE marking scheme.
7. What are the common conceptual errors students make regarding the kinetic theory and how to avoid them in board exams?
- Assuming real gases always behave ideally—remember exceptions at low temperatures or high pressures.
- Confusing average speed with root mean square speed or most probable speed—these are distinct and have different formulas.
- Ignoring that energy distribution applies only at thermal equilibrium—equipartition only holds for equilibrium scenarios.
- Neglecting the role of molecular size and interactions in determining mean free path and deviations from ideality.
8. How does the kinetic theory explain Boyle’s and Charles’ laws, and what is the expected derivation format for exams?
- For Boyle’s Law (constant temperature), derive from kinetic theory that PV = constant due to pressure’s dependence on molecular collisions.
- For Charles’ Law (constant pressure), show that V ∝ T as kinetic energy and molecular velocities increase with temperature.
- Answer should include use of derived equations and clear stepwise explanation as per 2025–26 CBSE guidelines.
9. How do you calculate the root mean square (rms) speed of gas molecules, and why do different gases at the same temperature have different rms speeds?
RMS speed, crms = √(3kT/m) for each molecule or √(3RT/M) for one mole, where m is molecular mass and M is molar mass. At the same temperature, lighter gases have higher rms speeds because speed is inversely proportional to the square root of mass.
10. What topics from Kinetic Theory are frequently marked as 'most expected' or 'high weightage' for board exams as per recent CBSE patterns?
- Full derivation of pressure of ideal gas (5 marks)
- Numericals on rms, average, and most probable speeds
- Definition and calculation of mean free path
- Law of equipartition of energy with examples
- Comparisons between ideal and real gases, and derivations of gas laws from kinetic theory
11. How do equipartition of energy and degrees of freedom determine the specific heat capacities of monoatomic and diatomic gases? (HOTS exam focus)
Specific heat at constant volume, Cv, is proportional to the number of degrees of freedom (f): Cv = (f/2) R. For monoatomic gases (f=3), diatomic gases (f=5 at room temperature), this explains their varying specific heats. This relationship is a favorite for deeper conceptual and application-based exam questions.
12. If two gases have different molecular masses but are at the same temperature and pressure, will they have the same number of molecules and kinetic energy? Explain.
At same temperature and pressure, equal volumes of gases have the same number of molecules (Avogadro’s law). However, each molecule has the same average kinetic energy, but lighter molecules will move faster. Total kinetic energy per mole depends on temperature, not mass.
13. What are board-exam relevant numericals that often appear from the Kinetic Theory chapter? Cite at least two typical types.
- Finding rms speed, mean free path, or number of collisions per second for given conditions of gas and temperature.
- Application-oriented problems involving the behavior of real gases under changing temperature and pressure using the ideal gas equation, especially those involving conversion between Celsius and Kelvin or SI units.

























