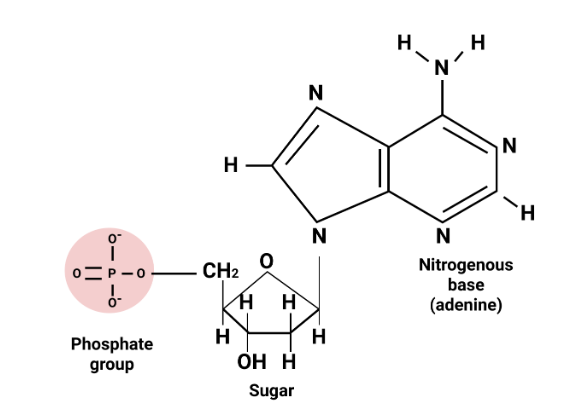
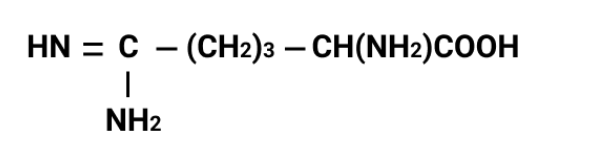Biomolecules Class 12 important questions with answers PDF download
FAQs on CBSE Important Questions for Class 12 Chemistry Biomolecules - 2025-26
1. What are the most commonly asked types of questions on biomolecules in the CBSE Class 12 Chemistry board exams?
Biomolecules questions frequently target definitions, structures, functions, and differences of key biomolecules such as carbohydrates, proteins, nucleic acids, and lipids. Students should also expect mechanism-based questions on enzymatic action and application-based questions involving vitamin deficiencies or the structural features of sugars and amino acids.
2. How should students prepare important questions on biomolecules for scoring maximum marks in the board exam?
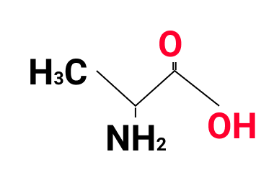
Focus on short answer formats and concise explanations aligned with mark-wise distribution. Prioritize revising one-mark factoids, two-mark differences, and three to five-mark application or HOTS (Higher Order Thinking Skills) questions. Practice writing precise answers for naming, drawing structures, and explaining key concepts like zwitterions or glycosidic linkage.
3. Why do CBSE exams frequently include questions about the functions of proteins and nucleic acids?
The exam tests understanding of core biological functions because proteins and nucleic acids play essential roles in living systems. Questions about DNA replication, protein synthesis, or enzyme action assess both factual knowledge and the ability to connect structure with function as outlined in the CBSE Chemistry syllabus.
4. What conceptual mistakes should be avoided while answering important questions on carbohydrates, such as reducing and non-reducing sugars?
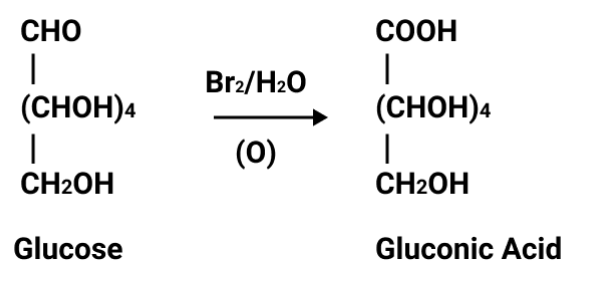
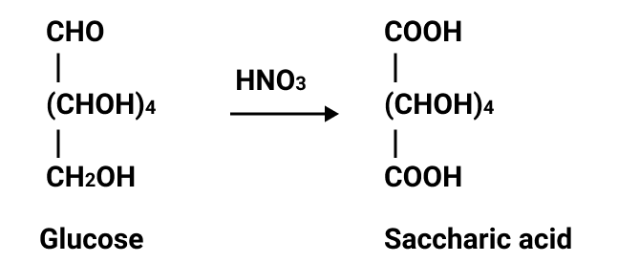
Students often confuse the structural features that determine reducing ability. Always mention the presence or absence of a free aldehyde or ketone group, not just the sugar’s classification. For instance, sucrose is non-reducing due to involvement of both anomeric carbons in glycosidic linkage, leaving no free group available for reduction.
5. Which biomolecules and related topics from Chapter 10 carry the highest weightage in CBSE Class 12 Chemistry important questions?
- Proteins: structure, types, and denaturation
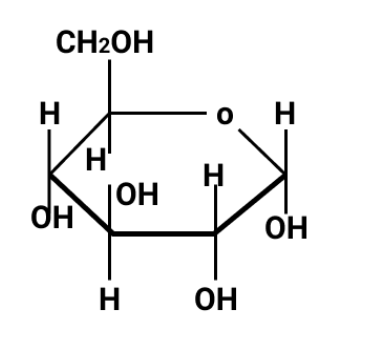
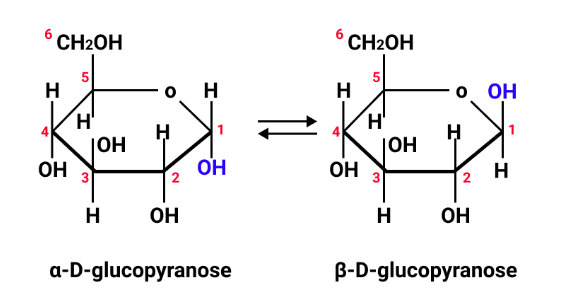
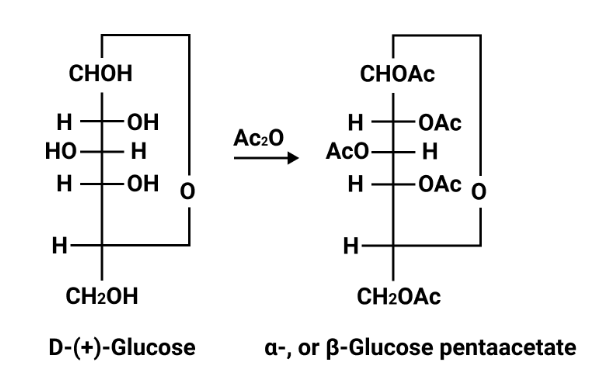
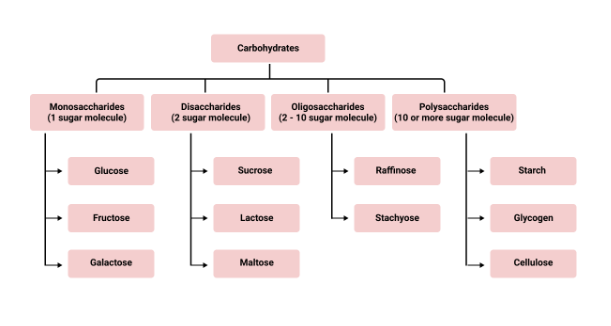
- Carbohydrates: classification, reducing/non-reducing sugars
- Amino acids: zwitterions, isoelectric point, peptide bond formation
- Nucleic acids: structure of DNA/RNA, base pairing
- Vitamins: classification, deficiency diseases
6. How can students effectively use important questions on biomolecules for last-minute revision before the board exam?
Begin by attempting one-mark direct questions and reviewing their precise answers. Move to HOTS and application-based questions to build confidence. Quick recaps with structure diagrams and definitions help reinforce memory, while practicing differences (e.g., glucose vs. fructose, globular vs. fibrous proteins) ensures readiness for comparative questions.
7. How do CBSE important questions ensure alignment with the 2025–26 Chemistry syllabus for Class 12?
Important questions are curated strictly as per official CBSE guidelines and syllabus updates. Expertise ensures all topics, especially those specified under biomolecules—such as enzyme characteristics, vitamin functions, and hereditary biomolecules—are covered in the expected mark-wise format for the particular board session.
8. What mistakes should students avoid when answering questions about enzyme action and specificity?
Avoid generic answers such as ‘enzymes are specific.’ Instead, clearly mention types of enzyme specificity (absolute, group, linkage) and relate the concept to structure-function relationships. Use examples, such as how maltase specifically hydrolyzes maltose, to convey clarity and depth expected by CBSE evaluators.
9. Why are HOTS (Higher Order Thinking Skills) questions about biomolecules significant for exam preparation?
HOTS questions challenge students to apply, analyze, or synthesize knowledge—going beyond rote memorization. These questions often test understanding of biochemical processes, structural differences, and interpretation of data or diagrams, helping students develop deeper comprehension and perform better in board exams.
10. How can practicing previously asked important questions help predict or prepare for expected CBSE board exam questions in chemistry?
Regular practice of past important questions exposes students to recurring patterns, frequently tested concepts, and answer styles preferred by the board. This strategic practice highlights high-weightage topics and typical examiner preferences, improving performance by familiarizing students with likely question formats and examiner expectations for the year 2025–26.