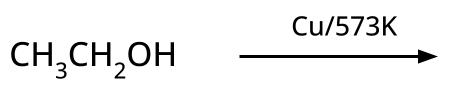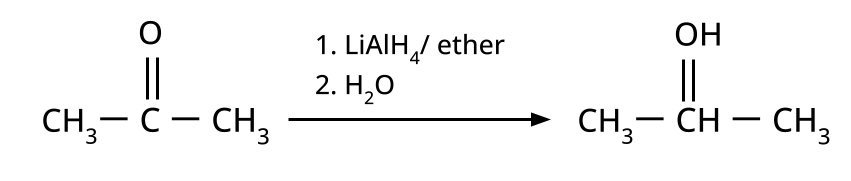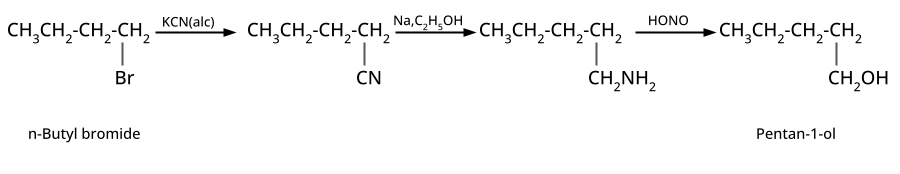Alcohols, Phenols and Ethers Class 12 important questions with answers PDF download
FAQs on CBSE Important Questions for Class 12 Chemistry Alcohols, Phenols and Ethers - 2025-26
1. What are the most important topics to focus on in Alcohols, Phenols, and Ethers for CBSE Class 12 Chemistry exams?
- Nomenclature and Classification: Correct naming rules for alcohols, phenols, and ethers.
- Preparation Methods: Key reactions such as hydration of alkenes, hydrolysis of ethers, preparation from halides, Kolbe's reaction, etc.
- Properties: Physical (boiling/melting points, solubility) and chemical (oxidation, esterification, substitution) properties.
- Reaction Mechanisms: Mechanisms of dehydration, oxidation, electrophilic substitution (phenols), Williamson ether synthesis.
- Acidity and Basicity: Comparative acidity/basicity of alcohols, phenols, and ethers with supporting reasons.
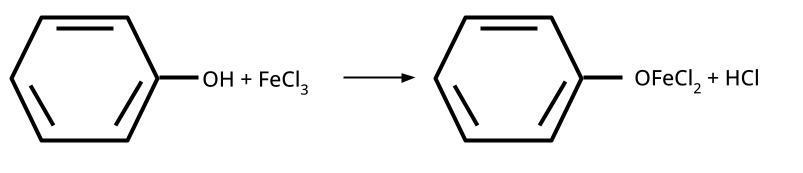
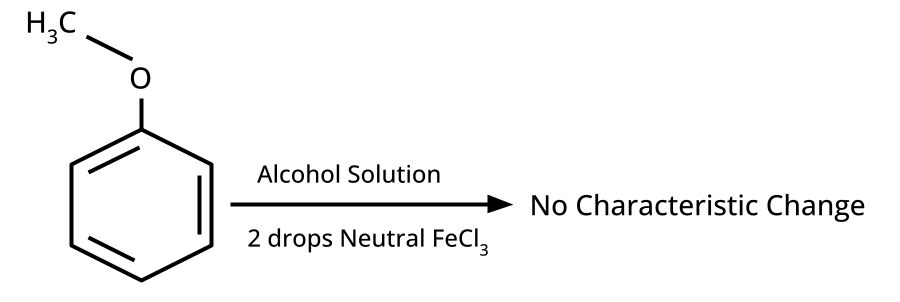
- Distinguishing Tests and Applications: Lucas test, iodoform test, FeCl3 test, etc.
2. Which types of questions from Alcohols, Phenols, and Ethers are frequently asked in CBSE board exams?
- One-mark: IUPAC nomenclature, definitions, short formulae.
- Three-mark: Reaction conversions, comparative acidity/basicity, explaining mechanisms.
- Five-mark: Mechanistic questions, multistep organic conversions, distinguishing tests for pairs of compounds, HOTS (Higher Order Thinking Skills) based conceptual applications.
Questions involving stepwise mechanisms, correct identification of reagents, and application of concepts to unfamiliar situations are especially important as per the latest CBSE (2025–26) pattern.
3. How is the acidity of phenol compared to alcohols and why is this question important for board exams?
Phenol is significantly more acidic than typical alcohols because the phenoxide ion formed after deprotonation is resonance-stabilized, dispersing the negative charge over the aromatic ring. In contrast, the alkoxide ion from alcohols does not have such stabilization, making alcohols less acidic. This concept is frequently tested by CBSE as it checks both reasoning and application of resonance concepts.
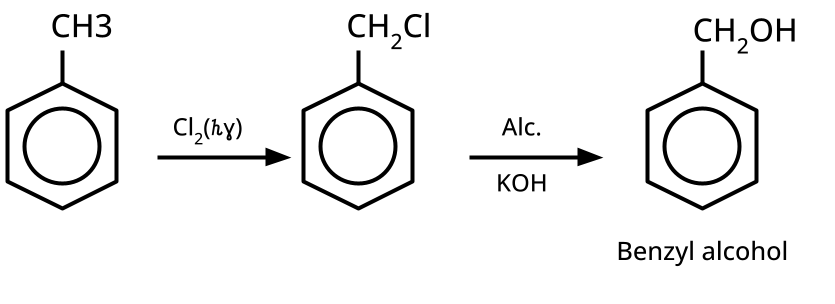
4. What are the key preparations of alcohols, phenols, and ethers that should be revised for CBSE exam short and long answers?
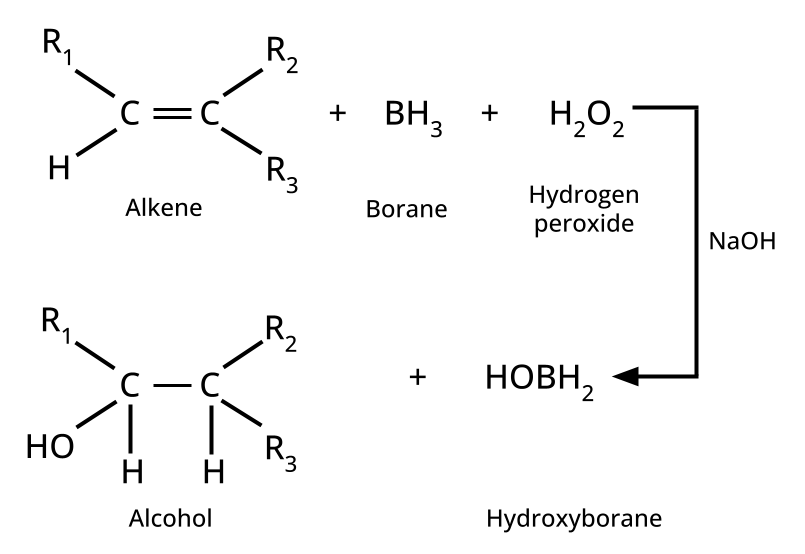
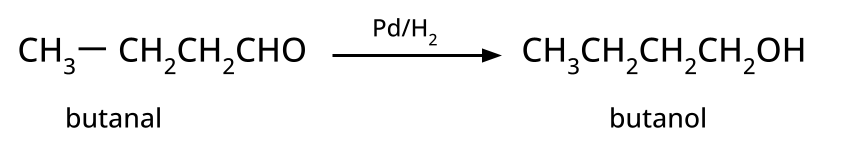
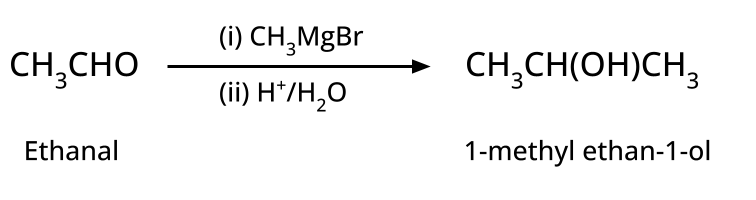
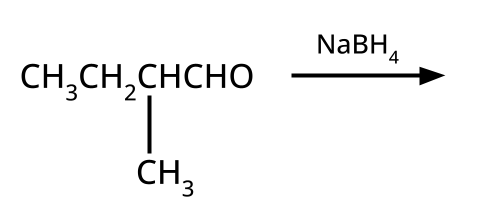
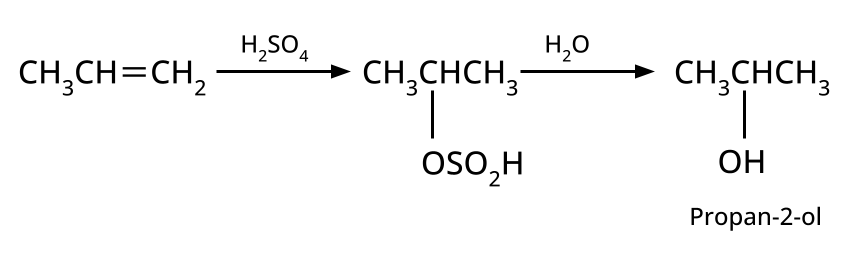
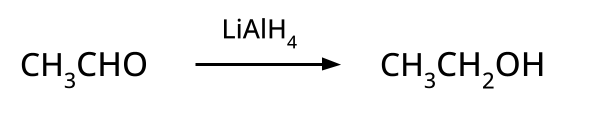
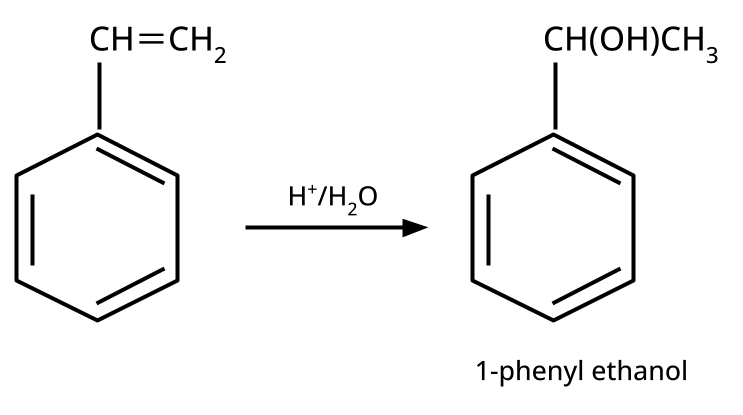
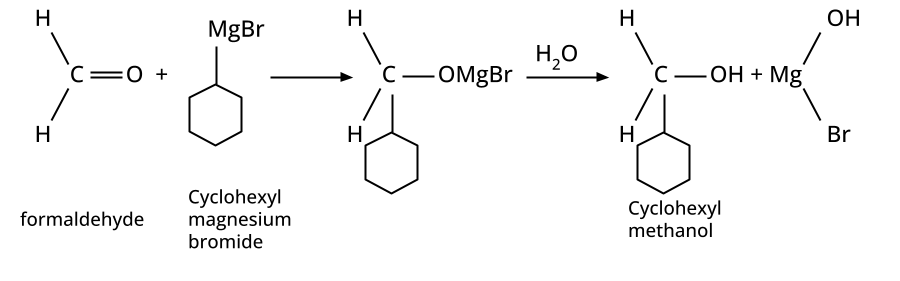
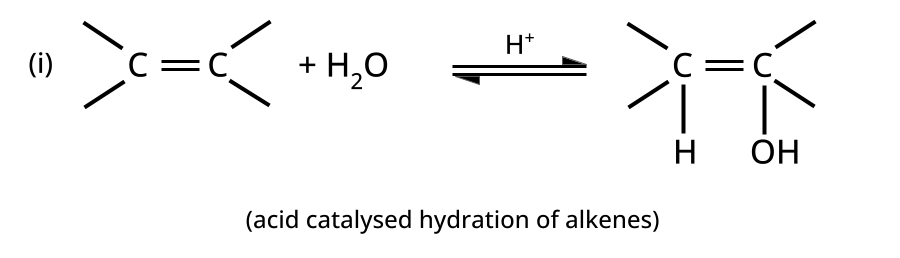
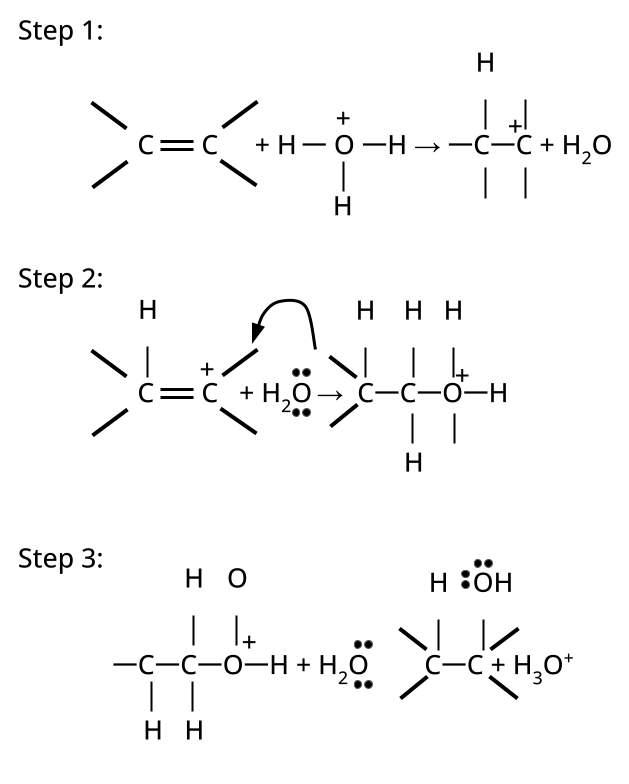
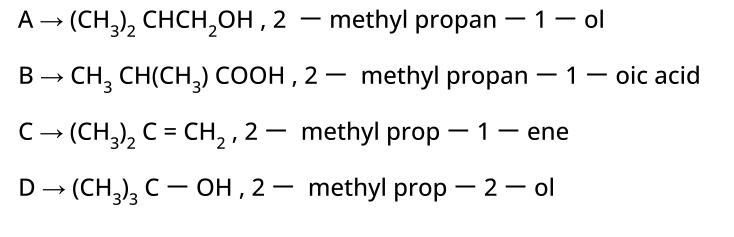
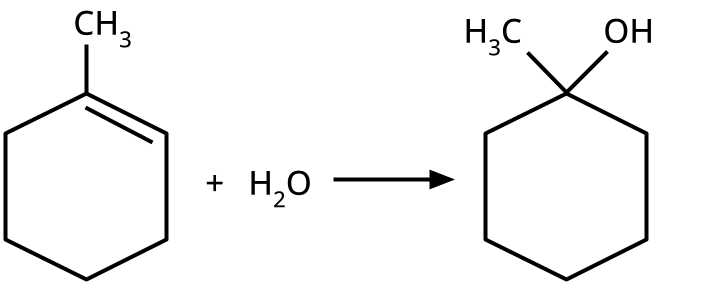
- Alcohols: Hydration of alkenes, reduction of carbonyl compounds, substitution of alkyl halides (SN1/SN2), hydroboration-oxidation.
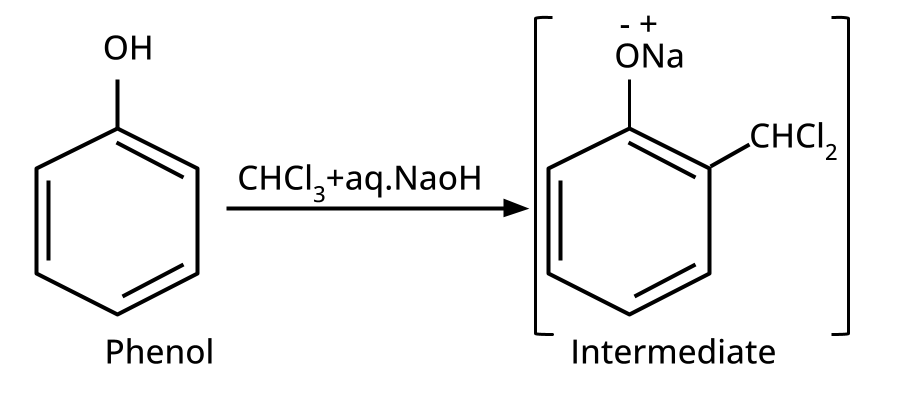
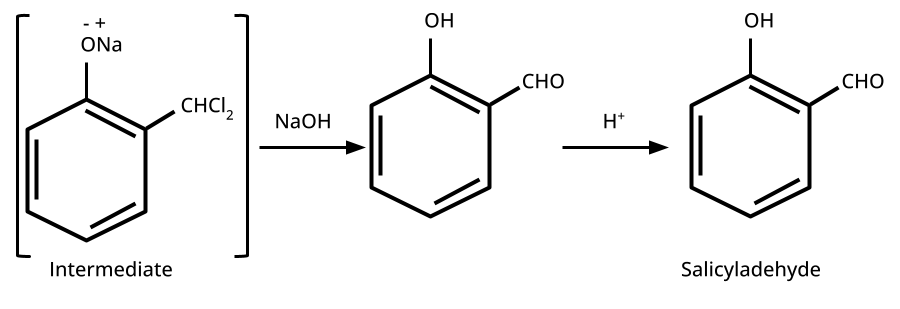
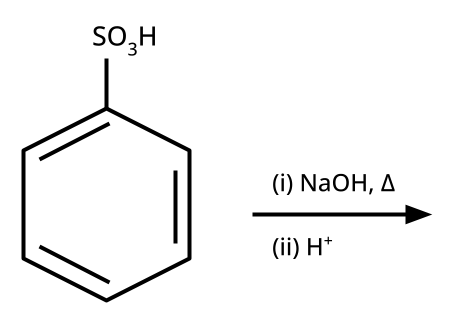
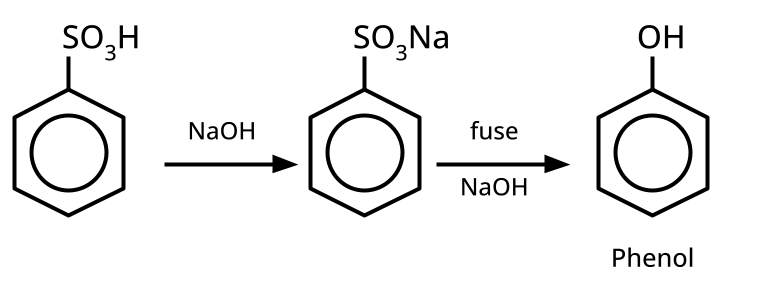
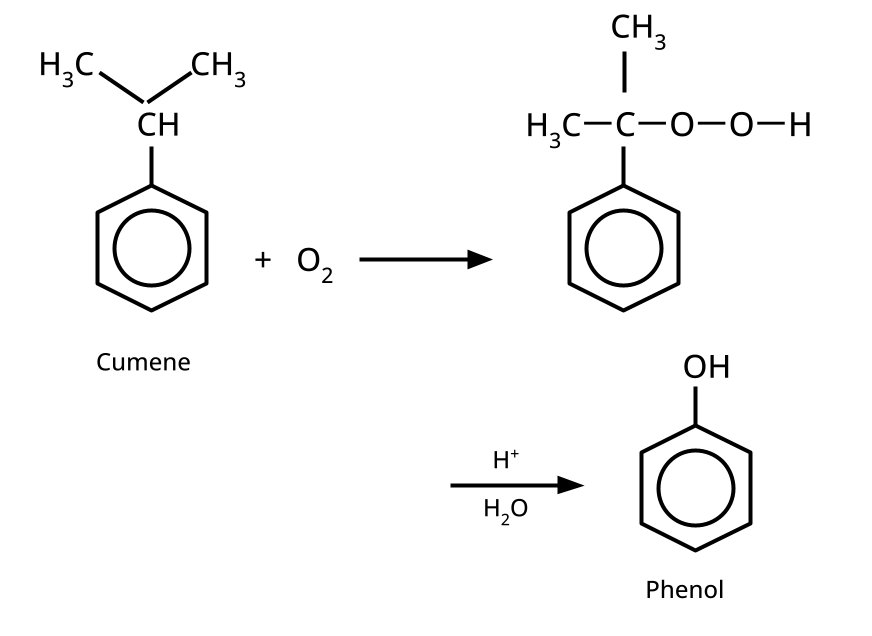
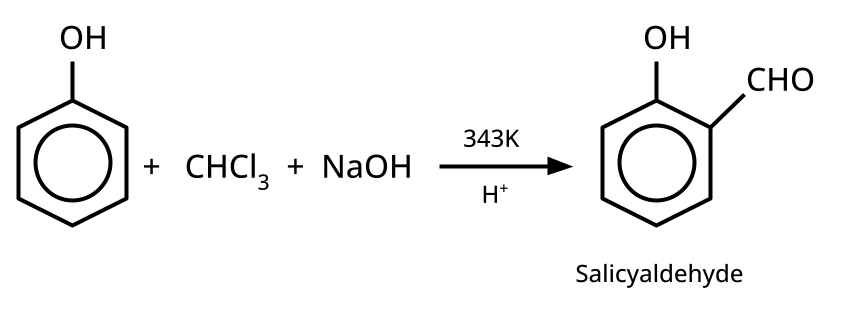
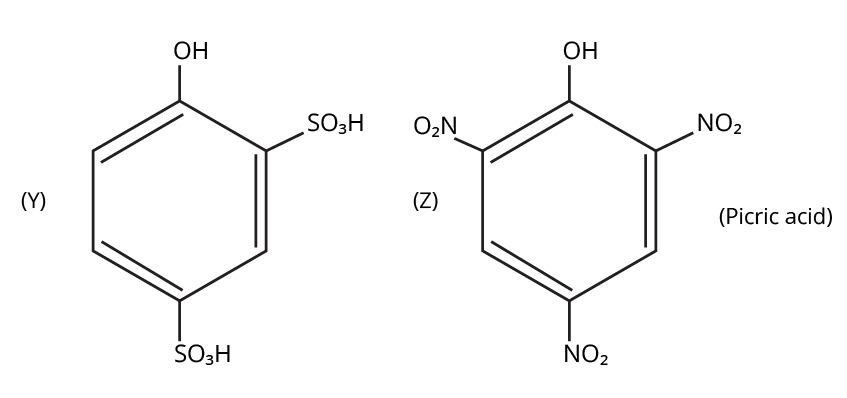
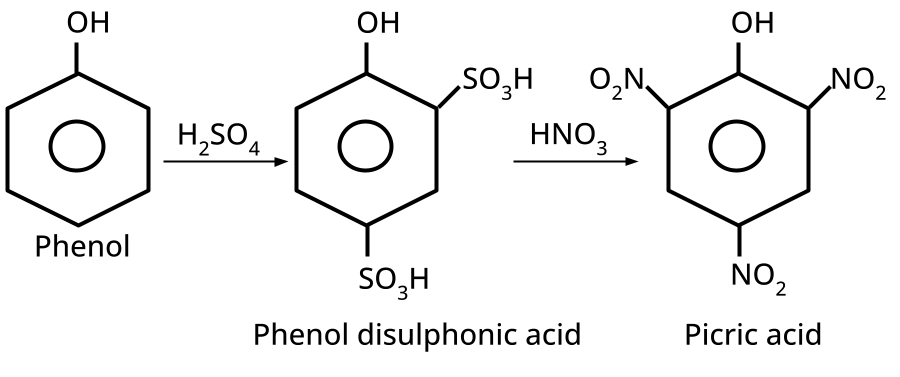
- Phenols: From diazonium salts (Sandmeyer reaction), from benzene sulphonic acid (alkaline fusion), from haloarenes (Dow's process).
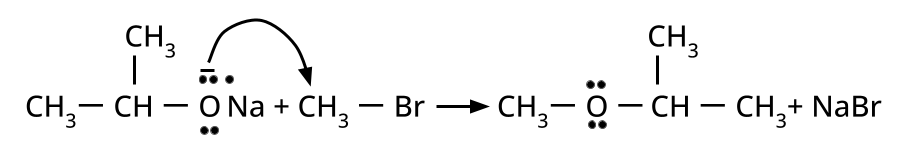
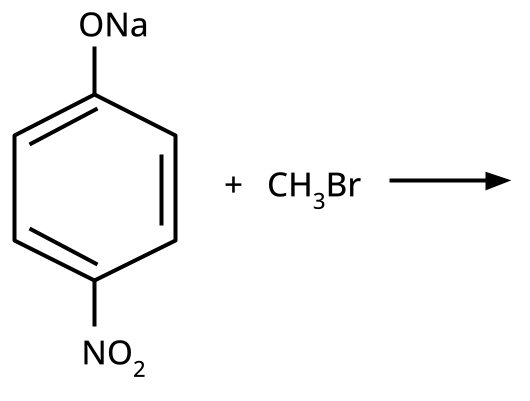
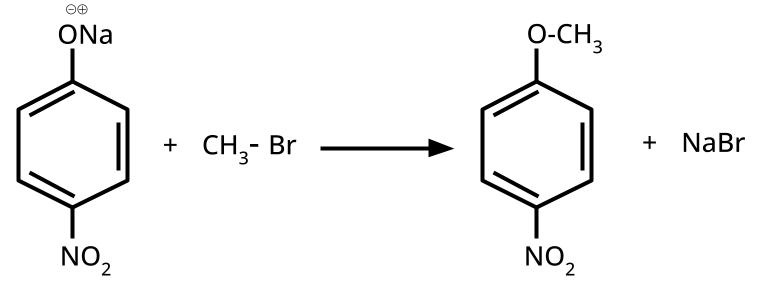
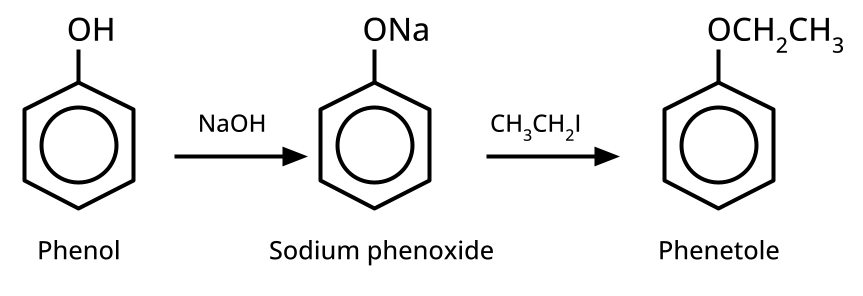
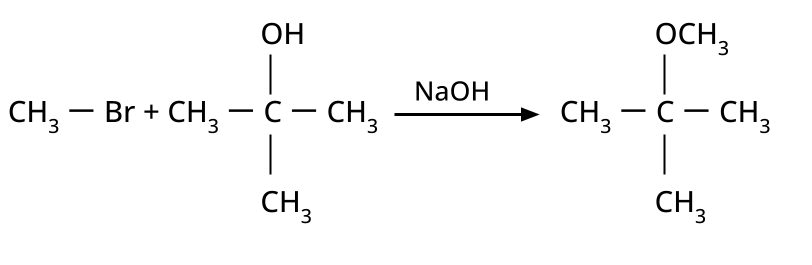
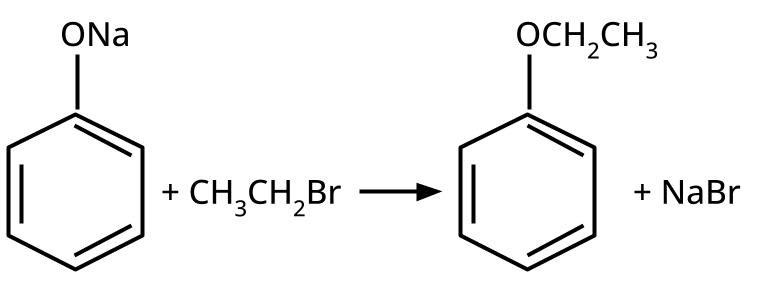
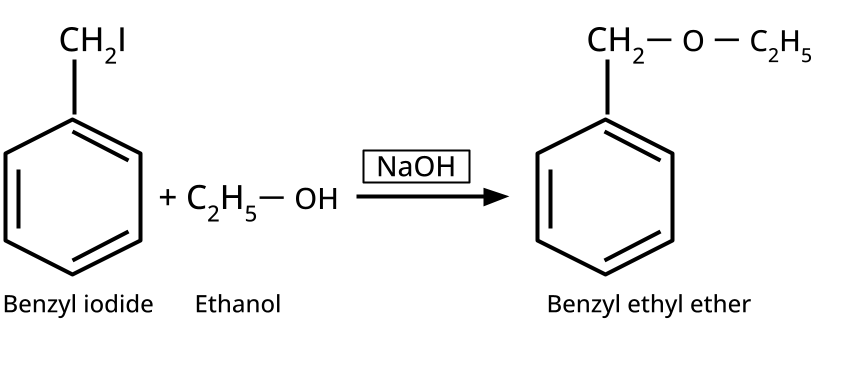
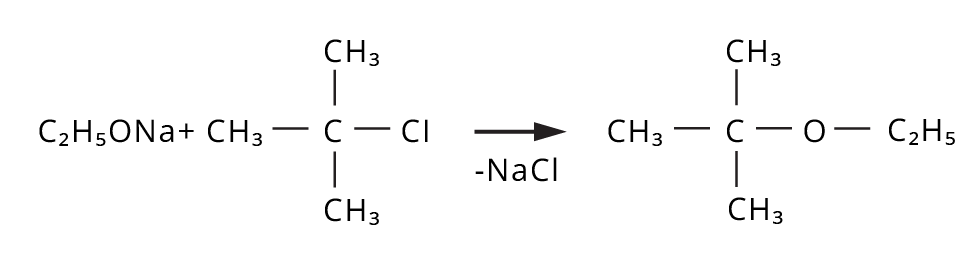
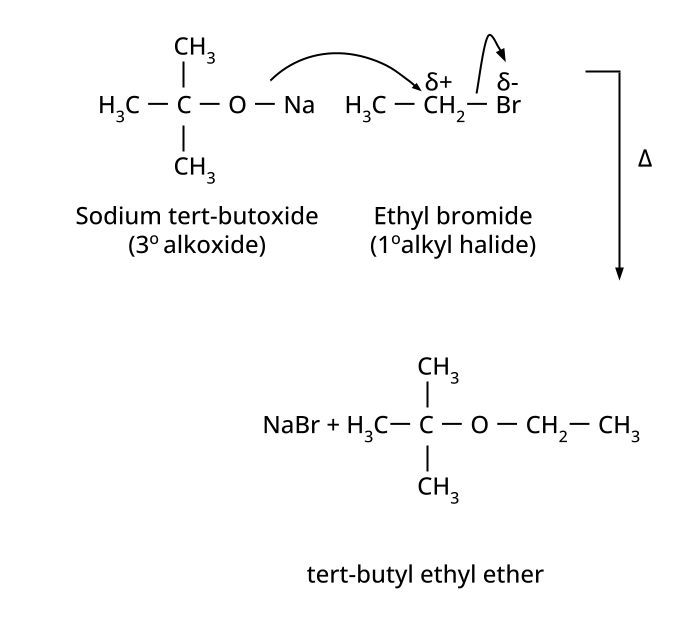
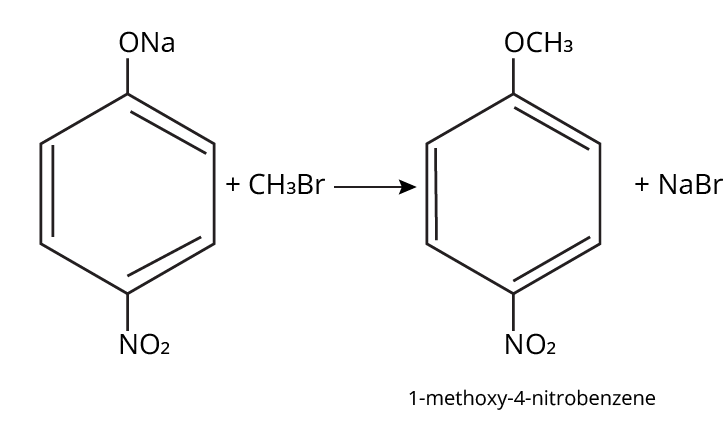
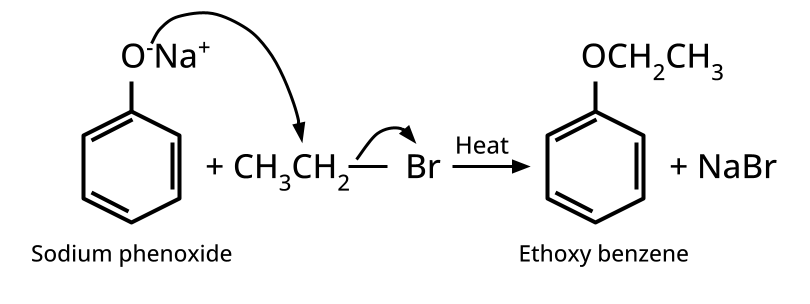
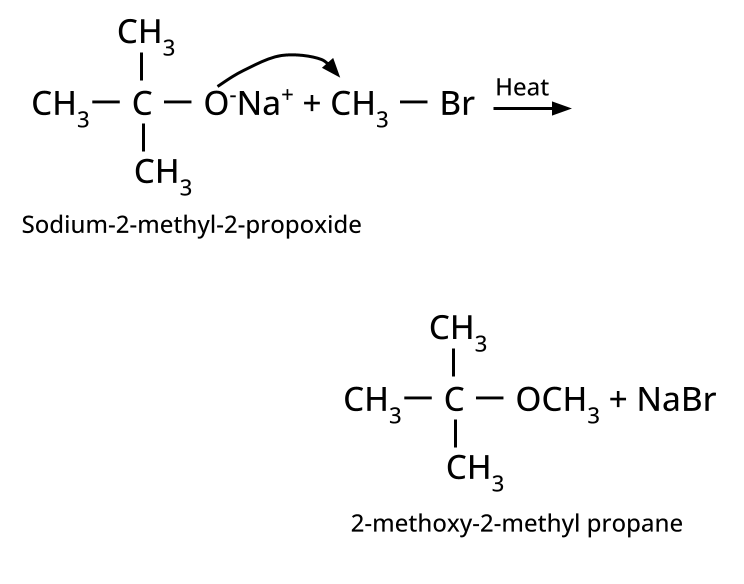
- Ethers: Williamson's synthesis (alkoxide + alkyl halide), dehydration of alcohols (symmetrical ethers).
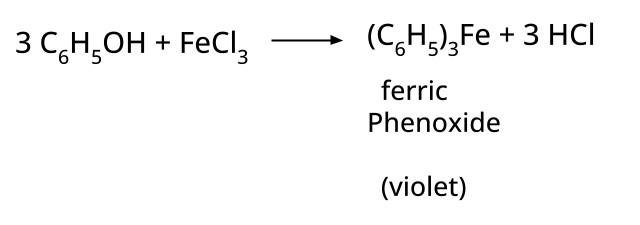
5. How to distinguish between phenol and ethanol using a chemical test, and why is this type of question repeatedly asked?
Phenol gives a violet coloration with neutral FeCl3 solution, while ethanol does not react. Such tests are commonly asked because they examine hands-on laboratory skills applied to organic functional group identification, a significant CBSE/NEET exam theme.
6. What mechanism-based question from this chapter is commonly tested as a 3- or 5-mark question in CBSE Chemistry?
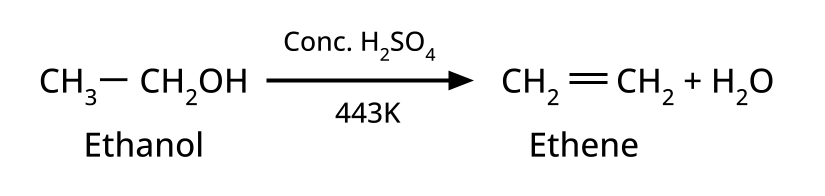
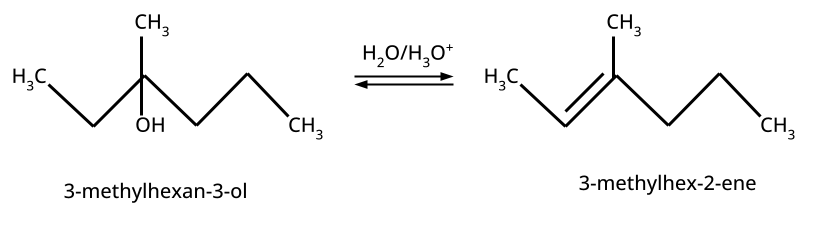
CBSE often asks for the mechanism of acid-catalyzed dehydration of alcohols to form alkenes (e.g., ethanol to ethene). Students must correctly illustrate carbocation formation, hydride shift (if applicable), and elimination steps, emphasizing the role of protonation and the stability of intermediates.
7. Which previous year question types from Alcohols, Phenols, and Ethers require conceptual rather than rote learning?
- Comparative questions (acidity, reactivity, boiling point trends, etc.)
- Application of reaction mechanisms to solve conversion problems
- Predicting major/minor products in reactions with explanations
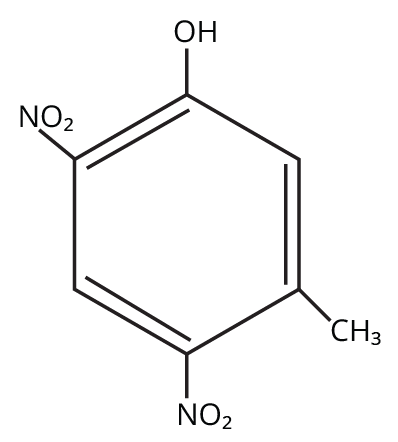
- Explaining anomalous behavior (e.g., steam volatility of o-nitrophenol vs p-nitrophenol)
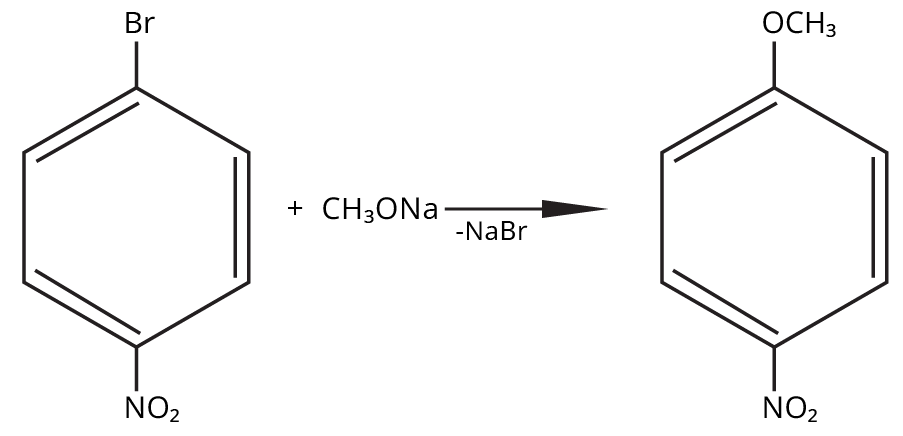
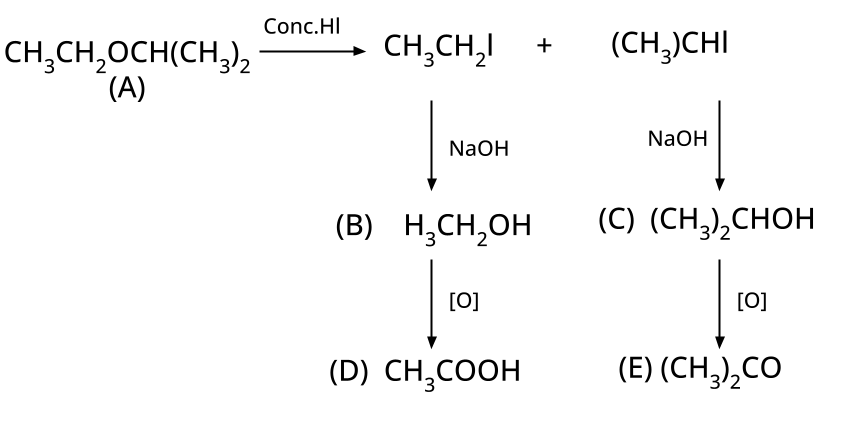
8. Why is the Williamson Ether Synthesis reaction not suitable for preparing aryl ethers using aryl halides? (Frequently Unsolved Question)
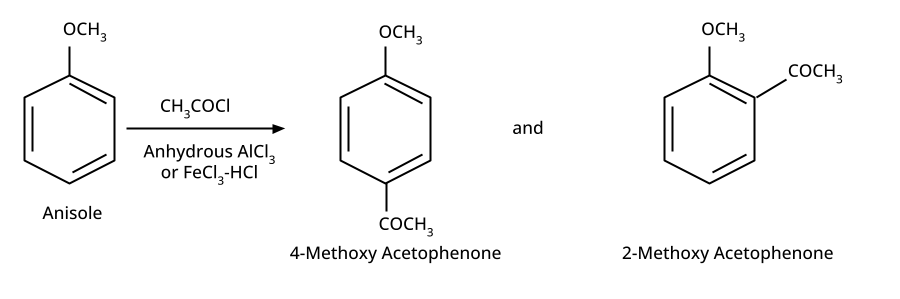
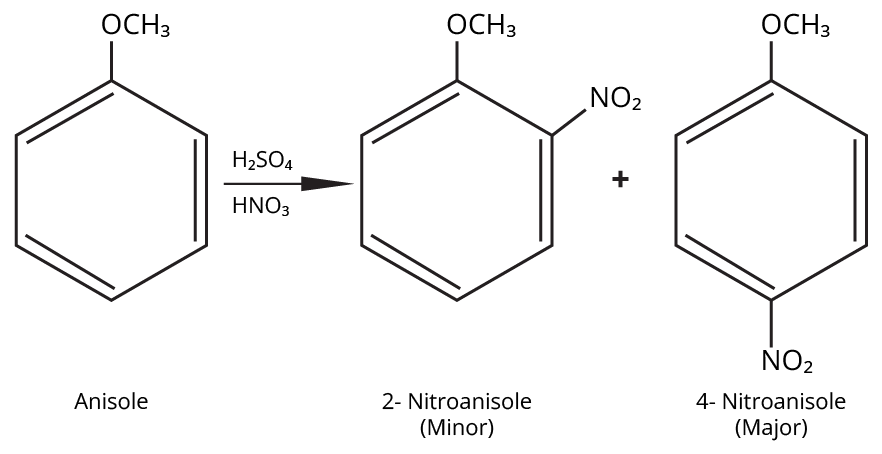
Aryl halides do not undergo nucleophilic substitution easily due to the partial double bond character of the C–X bond (resonance stabilization), making SN2 replacement exceedingly difficult. Thus, for aryl ethers like anisole, phenoxide ion is reacted with a suitable alkyl halide instead, not aryl halide.
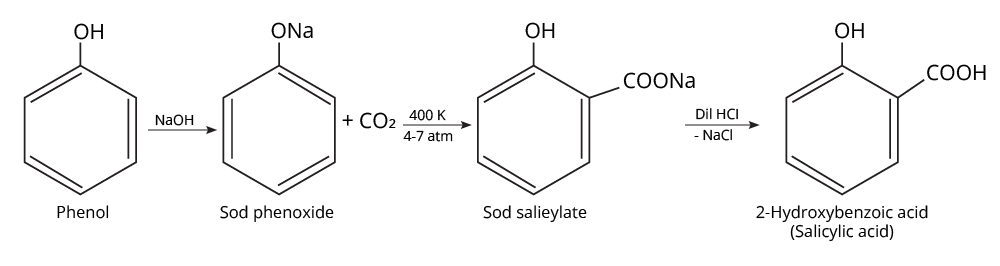
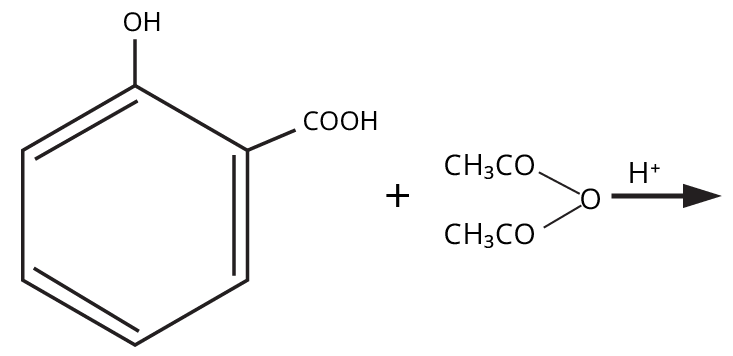
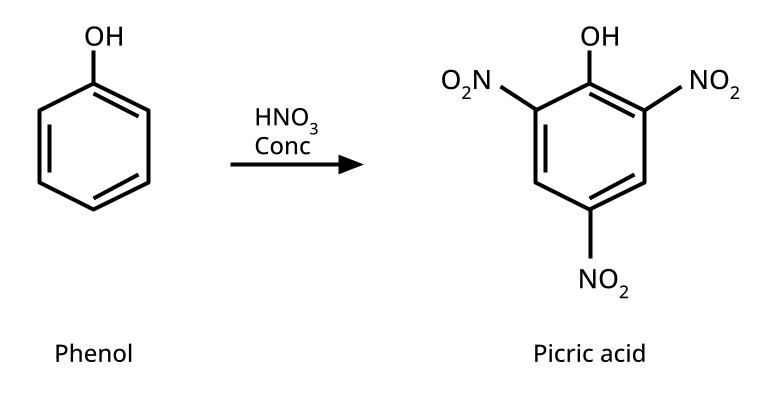
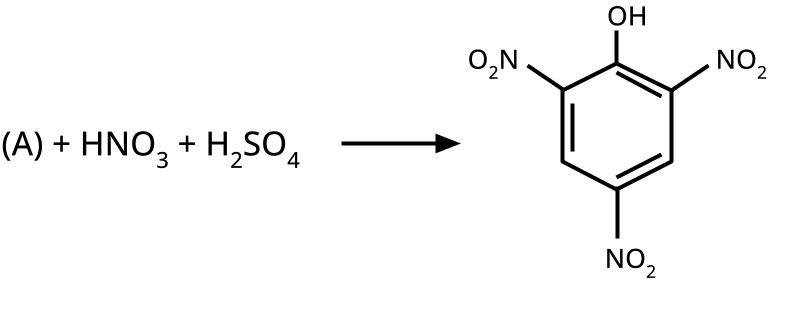
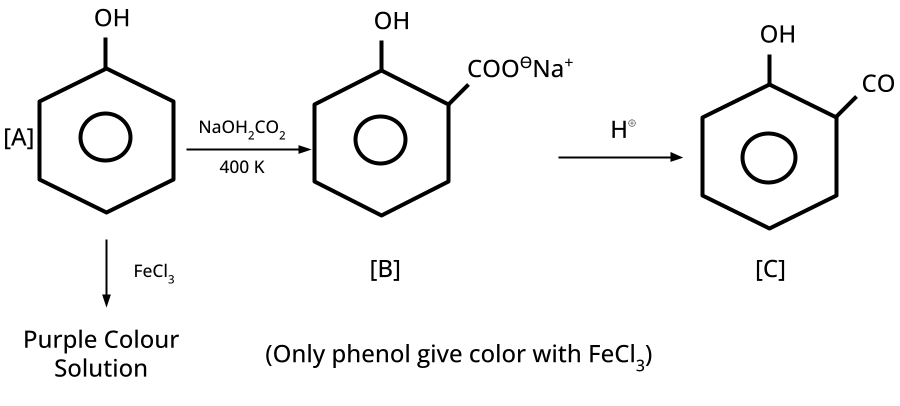
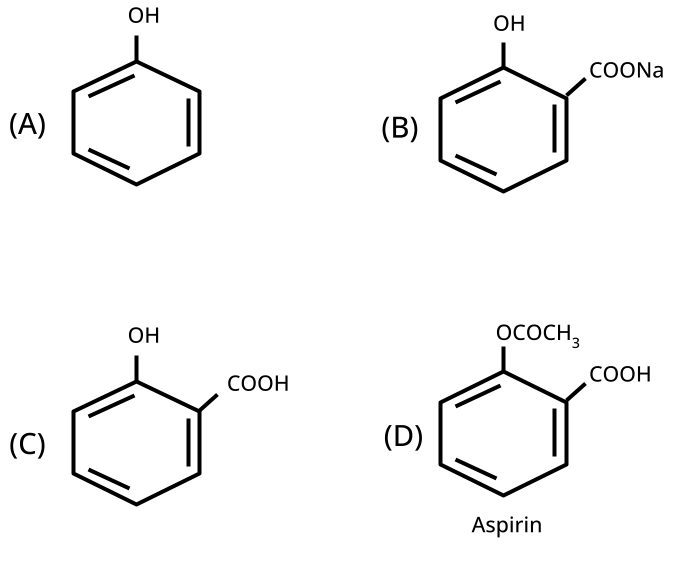
9. What is the board-exam significance of Kolbe’s Reaction in the context of phenols?
Kolbe’s Reaction demonstrates the formation of salicylic acid by reacting sodium phenoxide with CO2 under heat and pressure. It connects organic synthesis with industrial/commercial relevance, often asked as both a direct reaction and mechanism-based question—students must note reaction conditions for CBSE full marks.
10. How do the physical properties, especially boiling points, of alcohols, phenols, and ethers compare? Why is this a common CBSE question?
- Alcohols > Phenols > Ethers (for similar molecular mass)
11. What concepts are tested in CBSE questions when students are asked to arrange compounds in increasing/decreasing order of acidity or basicity?
Such questions assess students' grasp of inductive, resonance, and steric effects. For instance, the acidity trend like phenol < m-nitrophenol < o-nitrophenol < p-nitrophenol tests both resonance stabilization and the effect of electron-withdrawing substituents, a HOTS pattern often prioritized in board marking schemes.
12. What are common misconceptions students have about the reactions of alcohols and ethers on the exam?
- Assuming all alcohols react equally with sodium or Lucas reagent (secondary/tertiary alcohols react faster)
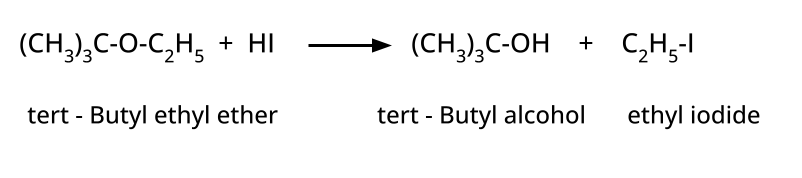
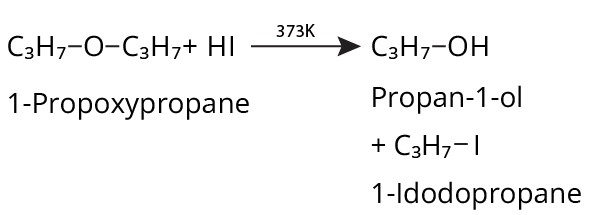
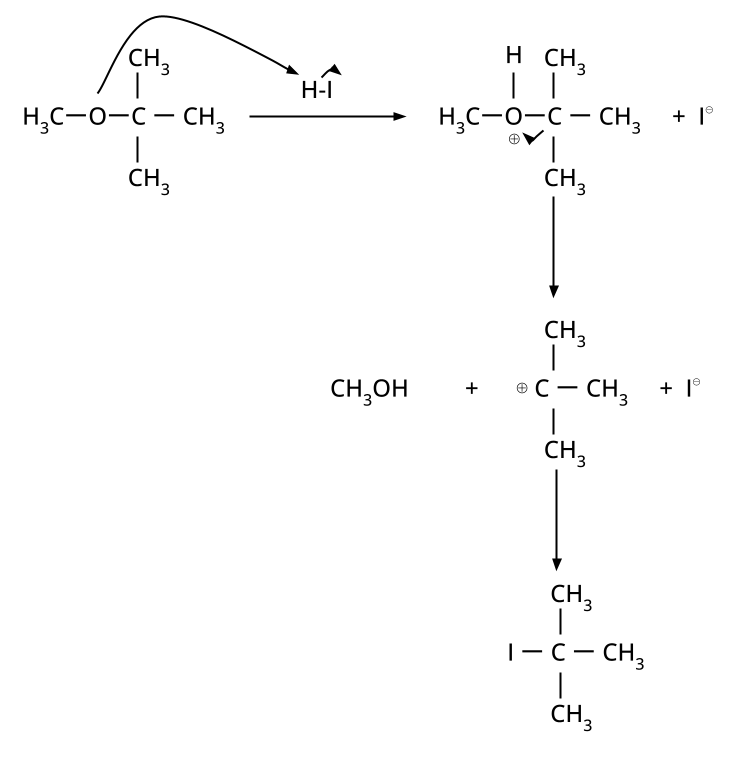
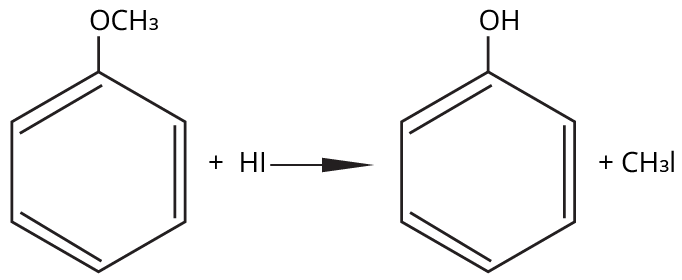
- Thinking all ethers react the same way with HI/HBr; in reality, cleavage patterns depend on structure
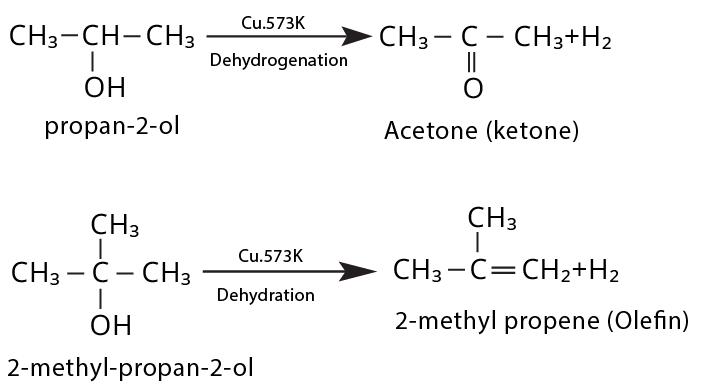
- Ignoring regioselectivity in dehydration/oxidation (placement of double bond or aldehyde/ketone group)
13. In practical-oriented questions, how might CBSE ask about distinguishing between primary, secondary and tertiary alcohols?
The Lucas Test is commonly cited. Primary alcohols react slowly/no visible turbidity at room temperature, secondary alcohols react within 5–10 minutes, and tertiary alcohols react immediately, producing turbidity due to alkyl chloride. The key is relating observations to molecular structure – a practical and theory combination.
14. Why is the esterification reaction considered a central reaction for Alcohols, Phenols, and Ethers in board and NEET exams?
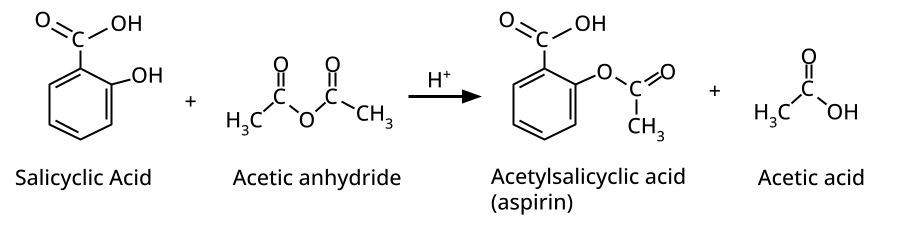
The esterification reaction (alcohol/phenol + acid → ester + water) demonstrates functional group transformation, mechanistic application (formation of tetrahedral intermediate), and general organic reactivity, linking the chapter to wider organic chemistry concepts. It's a high-yield reaction for conversion, MCQ, and mechanism-based questions.
15. What strategies should students follow to maximize marks in Alcohols, Phenols, and Ethers important questions for CBSE Class 12 (2025–26)?
- Prioritize reaction mechanisms and multi-step conversions over rote memorization.
- Practice explaining reasoning for acidity/basicity/comparative reactivity questions.
- Review and attempt HOTS and assertion-reason questions from sample and previous year papers.
- Use CBSE-recommended NCERT & exemplar questions for alignment with marking scheme trends.