Key Differences: Organic, Inorganic, and Physical Chemistry
Chemistry Formulas
FAQs on Essential Chemistry Terms Explained
1. What is an atom and what are its fundamental components?
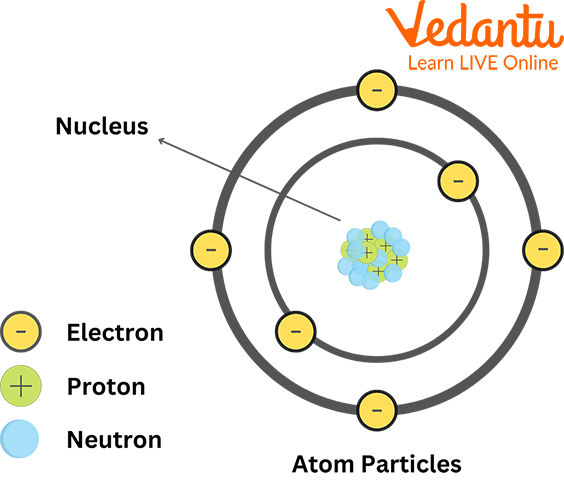
An atom is the smallest unit of an element that retains the properties of that element. It serves as the basic building block of all matter. The three fundamental subatomic particles that make up an atom are:
- Protons: Positively charged particles found inside the atom's nucleus.
- Neutrons: Neutral particles (no charge) also located in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus.
2. What is the main difference between an atom's atomic number and its mass number?
The key difference lies in what they measure. The atomic number (Z) represents the number of protons in an atom's nucleus. It is unique to each element and defines its identity. For example, any atom with 6 protons is a carbon atom. The mass number (A) is the total count of protons and neutrons in the nucleus, giving an approximation of the atom's total mass.
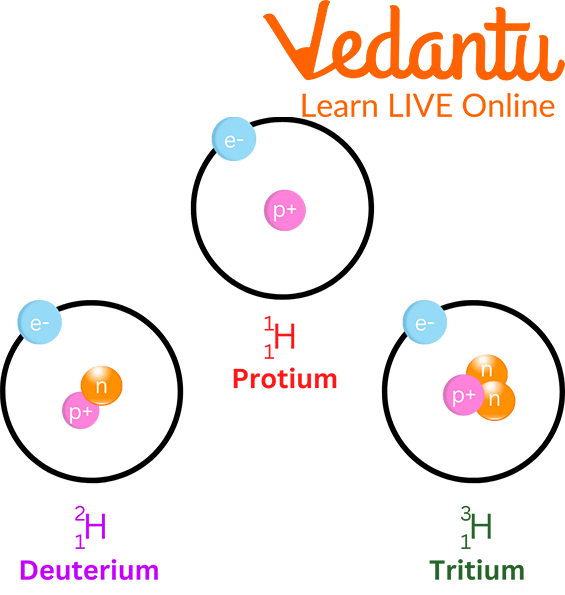
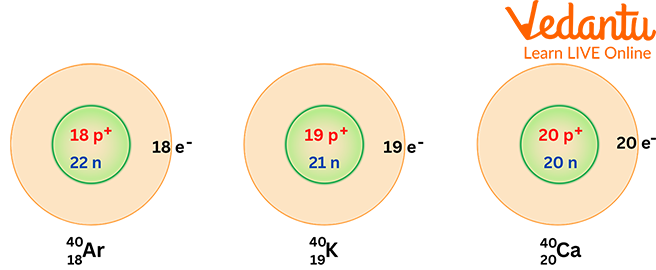
3. How do isotopes and isobars differ from each other?
Isotopes and isobars both describe relationships between different types of atoms, but they are opposites:
- Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons, resulting in a different mass number. For example, Carbon-12 and Carbon-14 are isotopes of carbon.
- Isobars are atoms of different elements (different atomic number) that happen to have the same mass number. For example, Argon-40 and Calcium-40 are isobars.
4. What are the essential differences between a compound and a mixture?
A compound and a mixture both contain multiple substances, but they differ in how those substances are combined. A compound is a pure substance formed when two or more different elements are chemically bonded together in a fixed ratio, like water (H₂O). A mixture consists of two or more substances that are physically combined without a chemical reaction and can be in any ratio, like salt dissolved in water.
5. Why isn't the mass of electrons included when calculating the mass number of an atom?
The mass of an electron is extremely small compared to that of a proton or a neutron. A proton or neutron is approximately 1,836 times more massive than an electron. Because their contribution to the total atomic mass is so insignificant, the mass of electrons is considered negligible and is omitted from the mass number (A) calculation for simplicity and practicality.
6. How can isotopes of the same element have different masses but identical chemical properties?
An element's chemical properties, such as how it reacts with other elements, are determined by its electron configuration, which is dictated by the number of protons in its nucleus (the atomic number). Since all isotopes of an element have the same number of protons and electrons, their chemical behaviour is virtually identical. The difference in neutrons only affects physical properties like mass and nuclear stability.
7. What are some real-world examples of an element, a compound, and a mixture?
We encounter these basic chemical forms every day. Here are common examples:
- Element: A piece of copper wire is made almost entirely of copper (Cu) atoms.
- Compound: Common table sugar, or sucrose (C₁₂H₂₂O₁₁), is a compound where carbon, hydrogen, and oxygen atoms are chemically bonded.
- Mixture: The air we breathe is a mixture of gases, primarily nitrogen (N₂) and oxygen (O₂), that are physically mixed but not chemically bonded.
8. What are the main branches of chemistry?
Chemistry is a vast field, typically divided into five main branches that help categorise different areas of study:
- Organic Chemistry: The study of carbon-containing compounds, which are the basis of life.
- Inorganic Chemistry: The study of all other compounds, including metals, minerals, and non-metals.
- Physical Chemistry: Focuses on the physical principles, like energy and quantum mechanics, that underlie chemical systems.
- Analytical Chemistry: The science of identifying and quantifying the chemical components of substances.
- Biochemistry: The study of chemical processes that occur within living organisms.
9. Why is chemistry often called the 'central science'?
Chemistry is referred to as the 'central science' because it provides a fundamental understanding of matter and its interactions, forming a crucial bridge between other scientific disciplines. It connects the fundamental laws of physics with the complex systems studied in biology, explains the composition of materials in geology, and is essential for developing new technologies in medicine and engineering.
10. Who is known as the father of modern chemistry and what was his key contribution?
Antoine Lavoisier is widely regarded as the father of modern chemistry. His most significant contribution was the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. He also established a systematic method for naming chemical compounds and demonstrated the role of oxygen in combustion, moving chemistry from a qualitative to a quantitative science.