




What Happens When Alcohol Reacts with Oxygen?
Alcohols are organic compounds, and they can be easily extracted from natural sources through the process of distillation. Alcohols are used to manufacture sanitiser, perfumes, and sweeteners and to prepare several chemical compounds. Ethyl alcohol is used as a solvent for many compounds, as it helps in dissolving polar, nonpolar, hydrophilic, and hydrophobic compounds as it acts as a universal solvent. On oxidation, alcohols produce aldehydes and ketones, which on further oxidation, produce carboxylic acid.
Water is a compound made of elemental hydrogen and oxygen. H2O is a chemical formula of water. It is a tasteless and odourless liquid. It is believed that the first life originated in water. It is one of the best solvents for inorganic compounds. To know more about alcohol and water, continue reading this article.
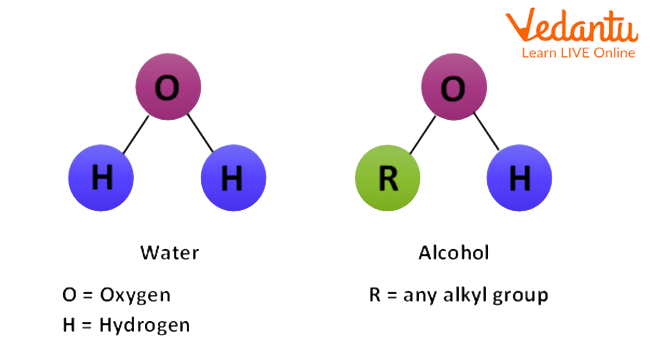
Molecular Structure of Water and Alcohol
Classification of Alcohol
Alcohols are mainly classified into primary, secondary and tertiary alcohols based on the chain of hydrocarbons (alkyl group) to the hydroxyl group attached.
1. Primary Alcohols
Primary alcohols are the alcohols in which only one alkyl substituent is attached to the carbon-containing hydroxyl (-OH) group; methanol also comes under this classification. The general formula of a primary alcohol is- R—CH2—OH, where R can be any alkyl group or hydrogen.
Examples of primary alcohols are-
CH3—OH (methanol)
CH3—CH2—OH (ethanol)
CH3— CH2— CH2—OH (propan-1-ol)
CH3— CH(CH3)— CH2— CH2—OH (3-methylbutan-2-ol)
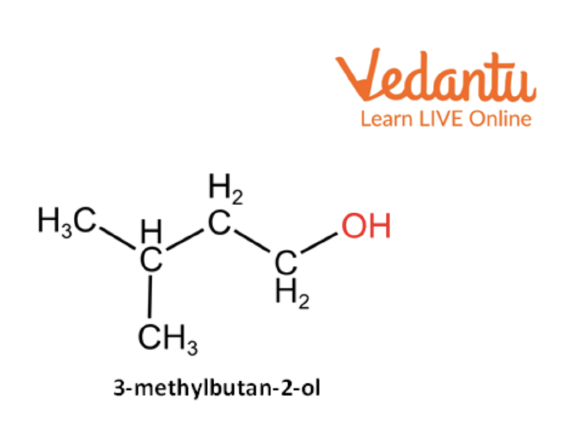
3-Methylbutan-2-ol
2. Secondary Alcohols
Secondary alcohols are the alcohols in which two alkyl substituents are attached to the carbon-containing hydroxyl (-OH) group. The general formula of secondary alcohol is- R2—CH—OH, where R can be any alkyl group.
Examples of secondary alcohols are-
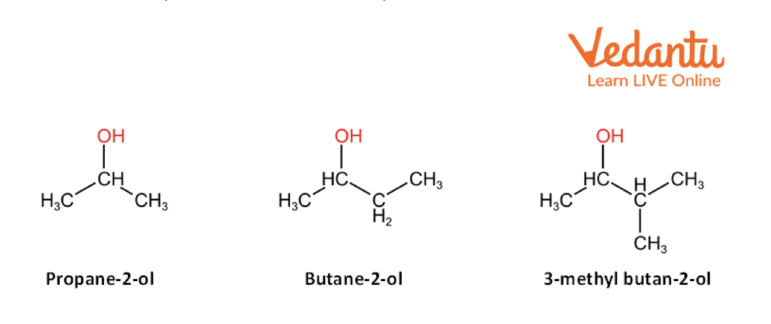
Secondary Alcohol
3. Tertiary Alcohols
Tertiary alcohols are the alcohols in which three alkyl substituents are attached to the carbon-containing hydroxyl (-OH) group. The physical properties of tertiary alcohols are primarily dependent on their structure. The general formula of tertiary alcohol is- R3—C—OH, where R can be any alkyl group.
Examples of tertiary alcohols are-
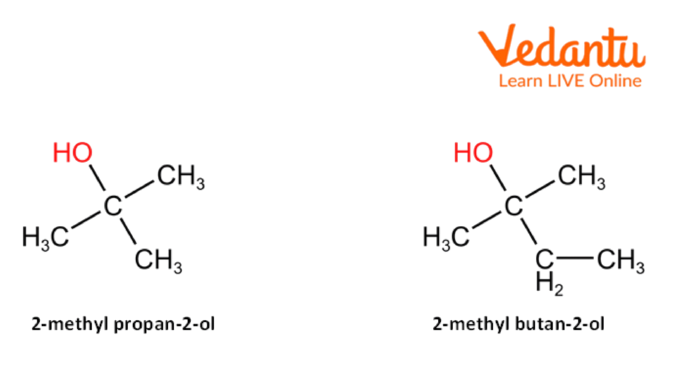
Tertiary Alcohol
4. Aromatic Alcohols (Phenols)
Phenols are the alcohols in which the hydroxyl (-OH) group is directly attached to the aromatic ring. Phenols are acidic due to the presence of an aromatic ring.
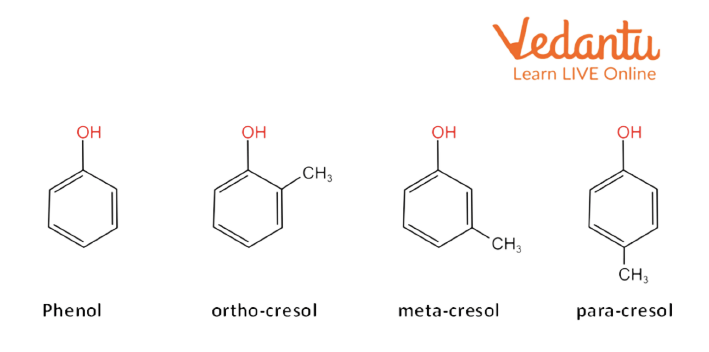
Aromatic Alcohol
Physical Properties of Alcohol
1. State: Generally, all alcohols are liquid at room temperature, but glycerol is a viscous liquid.
2. Colour: Alcohols are primarily colourless liquids.
3. Odour: Alcohols generally possess a sweet smell except for some lower alcohols and glycerol.
4. Boiling Point: They have a comparatively higher boiling point than other hydrocarbons of the same weight. Because intermolecular hydrogen bonding is present in the molecules of alcohol between the hydroxyl group. The boiling point of alcohol increases with the number of carbons in the hydrocarbon chain.
5. Flammability: Alcohols are flammable as they have a blue-coloured flame.
6. Nature: Alcohols act as weak acids as well as weak bases
7. Solubility in Water: Alcohols are soluble in water
8. Alcohol does not produce smoke while burning, and hence they, are also used as a fuel.
Physical Properties of Water
The following are some of the important physical properties of water:
Appearance: water is odourless, colourless and tasteless liquid.
Specific heat capacity of water: water has a high specific heat capacity due to extensive hydrogen bonds.
The density of water: The density of water varies with temperature.
The viscosity of water: The viscosity of water is 0.89 centipoise.
Surface tension: water has high surface tension.
Reaction of Ethanol with Air
When ethanol is burnt in the air it produces water and carbon dioxide.
CH3CH2OH(l)+3O2(g) → 2CO2(g)+3H2O(g)+Heat
Alcohols are highly flammable and are also used as a fuel.
When 2 moles of ethanol are burnt, carbon dioxide obtained is 4 moles and
When 2 moles of ethanol are burnt, CO2 obtained is 4 moles.
Interesting Facts
Drinking alcohol reduces the sugar level in the blood.
Drinking alcohol in small amounts can help us against heart disease, and it reduces the risk of heart disease.
Alcohol is used as a disinfectant and antiseptic, and it is also used in mouthwashes and sanitisers.
It is also used for the crystallisation of several compounds during laboratory preparations of these compounds.
Important Questions
1. Why is the boiling point of alcohol more than other hydrocarbons?
The boiling point of alcohol is more than other hydrocarbons because of hydrogen bonding in the molecules of the alcohols. Due to the high electronegativity of Oxygen, the shared pair of electrons shifts towards oxygen, creating a partial negative charge on oxygen and a partial positive charge on hydrogen. Which results in hydrogen bonding between hydroxyl groups of alcohol hence increasing the boiling point of alcohol.
2. What is glycerol?
Glycerol is a trihydroxy alcohol containing three hydroxyl groups on three different carbons of the same chain. The IUPAC name of glycerol is propane-1,2,3 triol. The structure of glycerol is-
Key Features
Alcohols are organic compounds containing at least one hydroxy (-OH) group. Alcohol burning in the presence of oxygen produces a large amount of energy, and hence alcohols are used as a fuel.
It can be used in chemical laboratories to form several compounds like carboxylic acid, aldehyde, ketone, alkene, and alkyl halides.
Alcohol is also a drug and is present in alcoholic beverages, which in smaller quantities is good for health, but in larger quantities, it is toxic. Alcohol is used as a sweetener in the food industry.
When ethanol is burnt in Oxygen it produces water and carbon dioxide.
FAQs on Alcohol and Oxygen Reaction Explained
1. What is the denaturation of alcohol?
When alcohols are adulterated with the addition of some chemicals to make them toxic, it is called the denaturation of alcohols. Mostly it is only done to make alcohol toxic for drinking. Denaturation of alcohol does not change any chemical or physical properties of alcohol. The alcohol is called denatured alcohol. The chemicals used for denaturation are benzene, gasoline, methanol, acetone, etc.
2. Which test is used to differentiate between primary, secondary, and tertiary alcohols?
The Victor Meyer test differentiates primary, secondary and tertiary alcohols. It is one of the most important tests to differentiate between primary, secondary, and tertiary alcohols. The process of the Victor Meyer test is-
Alcohol P+I2 → Iodoalkane alcoholic AgNO2→ Nitro Alkane Nitrous Acid (NaNO2&HCl) → Product(I) NaOH → Specific colour
The product (I) formed helps in differentiating the primary, secondary and tertiary alcohols. The product (I) formed by primary alcohol is Nitrolic acid which gives blood red colour with alkali (NaOH). The product (I) formed by secondary alcohol is Pseudo Nitrol which gives blue colour with alkali (NaOH). The tertiary alcohol does not react with Nitrous acid; hence, it gives no reaction and no colour.
3. Why is phenol acidic and toxic?
Phenol contains a benzene ring, and the benzene ring shows resonance; due to resonance, oxygen shares its lone pair of an electron in the resonance, and oxygen attracts the shared pair of electrons between O—H towards itself, making the hydrogen attached to oxygen very acidic. Hence the phenol is acidic and being acidic it is toxic as well.























