




How to Identify and Name Carbon Chains, Branches, and Rings
Hydrocarbons are compounds entirely made of carbon and hydrogen; the carbon in hydrocarbons forms long chains due to its catenating property. One carbon can, at most, form a bond with four other carbons, each of which can propagate the chain by linking to other carbon while satisfying its tetravalency.
The main classes of hydrocarbon chains are alkane, alkene, alkyne, cycloalkanes and arenes. Alkanes, alkenes, and alkynes are straight chains, while cycloalkanes and arenes are compounds with cyclic rings.
Carbon Chains
Straight chains of hydrocarbons can be branched or unbranched. The naming of hydrocarbon chains follows the IUPAC nomenclature, The name usually consists of a root, a prefix and a suffix. The nature of the skeleton- cyclic or acyclic- is indicated by the prefix, and the suffix indicates the type of bond or the functional group present in the chain. For branched compounds, the main carbon chain is identified and named; the location of the branching and the alkyl name is attached before the root (name of the main chain).
The carbon chain name rules for naming a branched hydrocarbon can be summed up as follows:
The longest continuous carbon chain is identified. This is called the main chain or the parent chain. It is named according to the number of carbon atoms in the chain.
Table: Roots of Basic Carbon Skeleton
Root | Number of Carbon atom |
Meth | One |
Eth | Two |
Prop | Three |
But | Four |
Pent | Five |
Hex | Six |
Hept | Seven |
Oct | Eight |
Non | Nine |
Dec | Ten |
In case of multiple chains with the same number of carbons, the chain with the maximum number of branching is selected as the parent chain.
The main chain is numbered in such a manner that the carbon atom to which the branches are attached gets the lowest possible number.
The name of the alkyl group attached to the carbon chain is placed before the name of the main chain. If the chain contains multiple similar branches, a prefix is added to indicate the number of branches. Some common prefixes are: di(2), tri(3), tetra(4), penta (5), hexa (6), hepta(7), and octa (8).
In case of different alkyl groups, the names are arranged in alphabetical order. The alkyl group derives their name from their base structure.
Table: Names of Alkyl Side Chains
Alkyl | Structure |
Methyl | —CH3 |
Ethyl | —CH2—CH3 |
Propyl | —CH2—CH2—CH3 |
Iso-propyl | 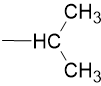
|
Butyl | —CH2—CH2—CH2—CH3 |
Iso-Butyl | 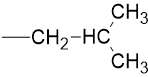
|
Tert-Butyl | 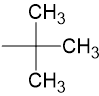
|
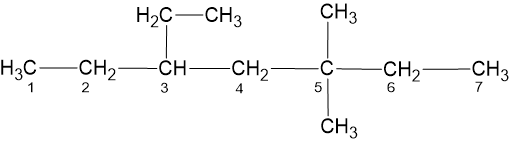
3 - Ethyl, 5, 5 - Dimethyl Heptane
If the branched chain contains multiple bonds, then the numbering is done according to the location of the multiple bond. The unsaturation receives the lowest number possible.
Table: Suffixes for Carbon Chain
Carbon Chain | Suffix |
Saturated | Ane |
Unsaturated: Double Bond | Ene |
Unsaturated: Triple Bond | Yne |
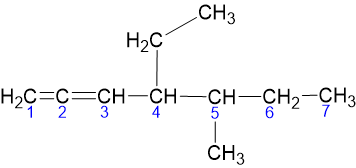
4 - Ethyl - 5 - Methyl - 1, 2 - Heptadiene
Hydrocarbons containing functional groups are named such that the carbon atom to which it is attached gets the lowest number possible. Halogen groups are prefixed before the root name of the main skeleton.
Table: Suffixes for Functional Groups
Organic Compound | Functional Group | Suffix |
Alcohol | -OH | -ol |
Aldehyde | -CHO | -al |
Ketones | >CO | -one |
-COOH | -oic acid | |
Acid Amides | -CONH2 | -amide |
Acid Chlorides | -COCl | -oyl chloride |
Esters | -COOR | -alkyl oate |
Cyanides | -CN | -nitrile |
Thioalcohols | -SH | -thiol |
-NH2 | -amine |
Table: Prefixes for Halogen Groups
Functional Group | Prefix |
Fluorine, F | Fluoro |
Chlorine, Cl | Chloro |
Bromine. Br | Bromo |
Iodine, I | Iodo |
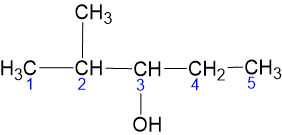
2 - Methyl - 3 - Pentanol
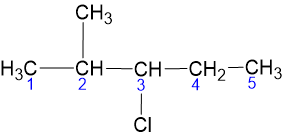
3 - Chloro - 2 - Methylpentane
Carbon Rings
Carbon atoms can bond with each other to form a variety of cyclic compounds- rings- which can be saturated or unsaturated. The cyclic alkane compounds are named with the word ‘cyclo’ set in the prefix, followed by the alkane name. For polycyclic hydrocarbons, the common name is accepted as IUPAC, owing to the compound's complexity. Aromatic rings have a high degree of unsaturation, and they form complicated architecture as a result their IUPAC nomenclature often becomes unreadable. The common name does not follow the IUPAC rules of naming. A few examples of aromatic compounds are mentioned below:
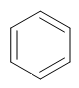
Benzene
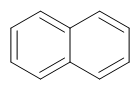
Naphthalene
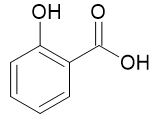
Salicylic Acid
Hydrocarbons containing multiple side chains attached to one cyclic system are considered derivatives of the cyclic compound. Rings with chains attached are named after the ring, and the substituted alkyl group names are attached as prefixes.
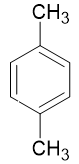
1, 4 - Dimethylbenzene
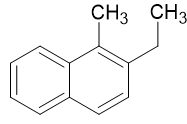
2 - Ethyl - 1 - Methylnaphthalene
Hydrocarbons with multiple chains and cyclic structure connected to one main chain is considered derivative of the acyclic compound.
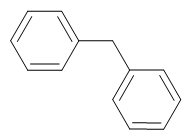
Diphenylmethane
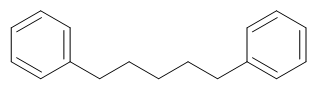
1, 5 - Diphenylpentane
Sometimes, two compounds with the same formula may have different structures, one of which is an open chain while the other is a ring chain. This type of isomerism is called Ring chain isomerism.
For example, Cyclopropane and propene both have the molecular formula C3H6.
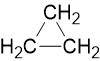
Cyclopropane

Propene
Interesting Facts
Biopolymers form the longest carbon chains.
Palytoxin and maitotoxin are marine compounds believed to have the longest carbon chain in nature.
Key Features
Alkane, alkene, alkyne, cycloalkanes and arenes are the main classes of hydrocarbons.
Carbon chains can be cyclic or acyclic.
Acyclic carbon chains can be branched or unbranched.
Carbon chains forming rings can be saturated: cycloalkanes or unsaturated arenes, also known as aromatic compounds.
FAQs on Carbon Chain, Branches, and Rings: Key Concepts Simplified
1. What is carbon tetravalency?
The uniqueness of carbon among all the elements owes to its electronic configuration. It has the atomic number 6. Two electrons fill the inner orbit, and four occupy the outer orbit. It requires four electrons to fill its octet, giving it a valency of four. It can form four covalent bonds at one-time, giving it the ability to form a large network of bonds.
2. What is a functional group?
Functional groups are substituents in molecules that form a part of a molecule and impart their unique chemical properties. Some examples of functional groups are the hydroxyl group (—OH), carbonyl group (—CO), aldehyde group (—CHO), and carboxylic acid group (—COOH).
3. What is an aromatic ring?
In modern chemical parlance, aromatic indicates delocalised bonding. Cyclic compounds that bear this type of delocalised bonding are called aromatic. The simplest aromatic compound is benzene (C6H6); it has a six-carbon ring with an alternate double and single bond, and the pi (double bond) electron in the ring is delocalised. The benzene ring is referred to as an aromatic ring, and compounds containing benzene are said to have aromatic rings.






































