




Essential Terms and Real-Life Applications of Charged Particles
The smallest particle of matter is an atom. In turn, there are three subatomic particles: protons, electrons, and neutrons. Protons and neutrons are the charged particles of an atom, and they are among the widely known and studied subatomic particles. Neutrons are negatively charged particles, whereas protons are positively charged.
For a long time, it was assumed that atoms are the ultimate particles of matter and that they cannot be further split. Experiments undertaken in the second half of the nineteenth and early twentieth centuries demonstrated that the atom is not the ultimate particle. Scientists' persistent efforts resulted in the discovery of subatomic particles.
The inability of Dalton's atomic hypothesis to explain certain data sparked the discovery of electrons and protons. Similarly, further research into neutrons was encouraged.
What Are Charged Particles in Matter?
Matter is any material with mass that occupies space. The smallest unit of matter is known as the "atom." With significant discoveries throughout the years, the structure of the atom was ultimately suggested, and it was verified that each atom contains charged particles or subatomic particles. In matter, these charged particles or subatomic particles are negatively charged electrons, positively charged protons, and neutral neutrons.
According to the most recent atomic structure, an atom is made up of a positively charged nucleus in the centre that is surrounded by electrons that rotate in various orbits around the nucleus. The positive charge of the nucleus is because of the positive protons. The structure of an atom is quite similar to that of our solar system, with the Sun at the centre resembling the nucleus and the planets moving in various orbits like electrons.
Types of Charged Particles in Matter
Three types of charged subatomic particles make up matter. They are as follows:
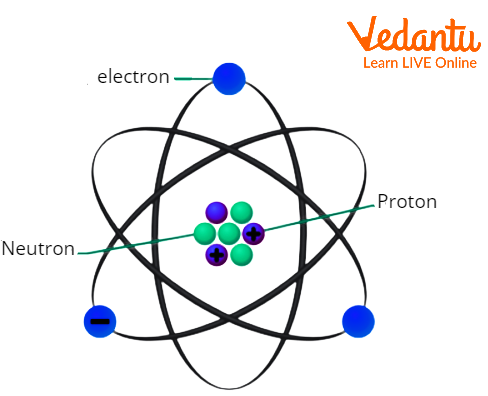
The Basic form of An Atom’s Structure
Electrons are negatively charged subatomic particles that exist in an atom. An electron has a charge of magnitude \[1.602\times {{10}^{-19}}\] Coulomb. An electron has 1:1837 the mass of a proton.
Protons are positively charged subatomic particles found in atoms. The charge of a proton is identical to the charge of an electron in magnitude; the charge is positive. A proton has a mass of \[1.673\times {{10}^{-27}}Kg\]. A proton is denoted by an H+ ion which is the nucleus of a hydrogen atom without an electron.
Neutrons are neutral subatomic particles found in all atomic nuclei. It has no electric charge at all and a mass of \[1.673\times {{10}^{-27}}Kg\]. It's slightly heavier than a proton but around 1839 times heavier than an electron.
Positively Charged Particles in Matter
Protons are the positively charged particles that are present in the nucleus of an atom.
Protons are present in the same number as the electrons in an atom.
Ernest Rutherford is credited with discovering protons.
It has a mass of \[1.676\times {{10}^{-24}}\] grams.
It has a charge of \[+1.602\times {{10}^{-19}}\]Coulombs.
Negatively Charged Particles in Matter
Electrons are subatomic particles that are negatively charged.
All elements' atoms contain an equal number of electrons and protons.
J. J. Thomson is credited with discovering electrons since he was the first to precisely calculate an electron's mass and charge.
The mass of an electron is tiny when compared to the mass of a proton. It has a mass that is equivalent to \[1/1837\] times the mass of a proton.
Its charge is equivalent to \[-1.602\times {{10}^{-19}}\] Coulombs.
Neutrally Charged Particles in Matter
Neutrons are subatomic particles that are neutrally charged.
Due to the variation in the number of neutrons in their respective nuclei, the masses of two distinct isotopes of an element differ.
In 1932, James Chadwick discovered the neutron.
They were found during an experiment in which an alpha particle bombarded a thin sheet of beryllium.
A neutron has a mass of \[1.676 \times {{10}^{-24}}\] grams.
Interaction of Charged Particles
When charged particles interact, energy is transferred from the charged particles to the materials through which they move. When two charges that are similar connect, they repel one another; when two charges that are opposite interact, they attract each other.
Two forms of particle-particle interactions—collisions and long-lasting interactions when particles are packed—are significant in the majority of applications. In actuality, internal stresses arise as a result of particle deformation during interaction.
Only a tiny portion of the energy of heavy charged particles may be transferred in a single collision. In a collision, it barely deflects at all. As a result, heavy charged particles move through matter virtually directly while continually losing energy in many collisions with atomic electrons.
Important Questions
1. Describe the term charged particles in matter.
Ans. A charged particle carries one that carries an electric charge. There are two kinds of electric charges: positive and negative. Two items with an excess of reputational force on each other.
2. What are matter's charged particles?
Ans. An electric charge exists in a charged particle. It might be an ion, such as a molecule or atom, having an excess or shortage of electrons in comparison to protons. It might also be an electron, a proton, or another primary particle, all of which are thought to have the same charge (except antimatter).
Practise Questions
1. Force due to magnetic field and velocity is
At right angles to one another
At an oblique angle to one another
At 180 degree angles to each other
Opposite to each other
Ans. At right angles to one another
2. Hall voltage is proportional to
Ans. Magnetic flux density
3. The force on a moving charge in a uniform magnetic field is determined by
Magnetic flux density
The charge on the particle
The speed of particle
All of above
Ans. Magnetic flux density
4. A charged particle is travelling perpendicular to the direction of a homogeneous magnetic field. Which of the following will change?
Speed
Velocity
Direction of motion
Both options 2 and 3
Ans. Both options 2 and 3
Summary
In conclusion, electrons, protons, and neutrons are the three charged subatomic particles that make up an atom. Later, the structure of the atom was established by several findings. A positively charged nucleus, created by the positively charged proton and neutral neutron at the centre of an atom, rotates around it like the solar system's orbiting electrons do. An atom is more stable because each electron in its fixed orbit around the nucleus has a distinct amount of energy. An electron's charge is $-1.602 \times 10^{-19}$, whereas the charge of a proton is approximately identical but is positively charged. The lightest subatomic particles are electrons, whereas protons and neutrons, which make up the majority of an atom's mass, are only found in the nucleus.
FAQs on Charged Particles in Matter Explained
1. What are the fundamental charged particles found in matter according to the CBSE Class 9 syllabus?
According to the syllabus for the 2025-26 session, matter consists of atoms, which contain two primary types of charged particles. These are protons, which carry a positive charge (+1), and electrons, which carry a negative charge (-1). Atoms also contain neutrons, but these are electrically neutral and have no charge.
2. Who were the key scientists credited with the discovery of the electron and proton?
The discovery of subatomic particles was a major scientific milestone. J.J. Thomson is credited with the discovery of the electron in 1897 through his experiments with cathode ray tubes. The proton was discovered by E. Goldstein in 1886 during his work on canal rays, which were found to be streams of positively charged particles.
3. Why is a typical atom considered electrically neutral even though it contains charged particles?
An atom is considered electrically neutral because it contains an equal number of protons and electrons. The total positive charge from all the protons in the nucleus is perfectly balanced by the total negative charge from all the electrons orbiting the nucleus. This balance results in a net charge of zero for the atom as a whole.
4. How do protons and electrons differ in their fundamental properties like mass, charge, and location within an atom?
Protons and electrons have distinct properties that define the structure of an atom:
Charge: A proton has a positive charge (+1), whereas an electron has an equal but opposite negative charge (-1).
Mass: A proton is significantly heavier than an electron. The mass of a proton is approximately 1,840 times the mass of an electron, making the mass of an electron almost negligible in comparison.
Location: Protons are located in the dense, central core of the atom, known as the nucleus. Electrons revolve around this nucleus in specific energy levels or shells.
5. What is a real-world example that demonstrates the presence of charged particles in everyday objects?
A common example is static electricity. When you run a plastic comb through dry hair, friction causes electrons to transfer from your hair to the comb. The comb becomes negatively charged. If you then bring this charged comb near small, neutral pieces of paper, it induces a positive charge in the paper, causing an attraction that makes the paper stick to the comb. This demonstrates how an imbalance of charged particles can create observable forces.
6. What are ions and how do they relate to the charged particles within an atom?
An ion is an atom or a group of atoms that has a net electrical charge. Ions are formed when a neutral atom either loses or gains one or more electrons. If an atom loses electrons, it has more protons than electrons, resulting in a positive charge (a cation). If an atom gains electrons, it has more electrons than protons, resulting in a negative charge (an anion).
7. Is there a state of matter that is primarily composed of charged particles?
Yes, the fourth state of matter, called plasma, is almost entirely made of charged particles. Plasma is a superheated gas in which the atoms have been stripped of their electrons due to immense energy. This creates a mixture of positively charged ions and free, negatively charged electrons. Stars, including our Sun, are giant balls of hot plasma.





































