




How to Identify Deactivating and Meta Directing Groups with Examples
In organic chemistry, a deactivating group (or electron withdrawing group) is a functional group attached to a benzene molecule that removes electron density from the benzene ring, making electrophilic aromatic substitution reactions slower and more complex relative to benzene. Depending on their relative strengths, deactivating groups also determine the positions (relative to themselves) on the benzene ring where substitutions must take place; this property is therefore important in processes of organic synthesis.
What are Meta Directing Groups?
If the relative yield of the ortho product and that of the para product are higher than that of the meta product, the substituent on the benzene ring in the monosubstituted benzene is called an ortho, para directing group. If the opposite is observed, the substituent is called a meta directing group. Example: Nitro group is a meta directing group.
What is Directive Effect?
For Ortho-para Directors
The resonance theory explains why some substituents are ortho-para and others are meta-directing. Let's look at some phenol resonance forms where the -OH group is already attached to the benzene ring.
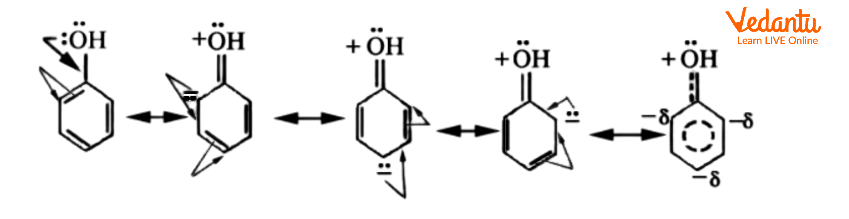
-OH Group Attached to Benzene Ring
Directive Effect
On the oxygen-atom of the -OH group attached to the ring, there are two nonbonding electron pairs. The interaction with the p-system distributes one of these into the ring. The ortho and para positions in resonance forms have a higher electron density than the meta positions. As a result of the electron delocalization, the resonance hybrid has negative charges in the ortho and para positions. Regardless of its nature, the electrophile (E+) would naturally gravitate toward these electron-rich centres.
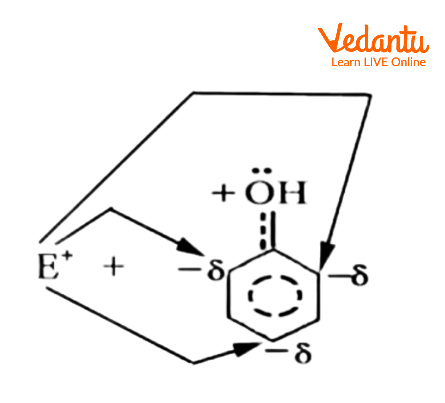
Electrophile Attracted Towards Electron-rich Centres
Are All Meta Directing Groups Deactivating or Not Deactivating?
They are meta-directing not because they stabilise the intermediate for meta-substitution: far from it. Instead, they destabilise the:
o,p-substitution intermediates in relation to the meta intermediate.
In general, o,p-directing groups stabilise o,p substitution intermediates relative to the meta intermediate.In comparison to the meta intermediate, m-directing groups destabilise the intermediates for o,p substitution.
What is Benzene?
Benzene is the most fundamental organic, aromatic hydrocarbon. Benzene is a basic petrochemical and a natural component of crude oil. It is a colourless liquid with a gasoline-like odour. In nature, benzene is highly toxic and carcinogenic. Its primary application is in the manufacture of polystyrene.
Although benzene is a naturally occurring substance produced by volcanoes and forest fires and found in many plants and animals, it is also a major industrial chemical derived from coal and oil. Benzene is a clear, colourless liquid when pure. Benzene is used in the manufacturing of other chemicals, as well as some plastics, detergents, and pesticides. It is also found in gasoline.
Important Questions
1. How will you account for the structure of benzene?
In benzene, all six carbon atoms are SP2 hybridised. Each carbon atom's two SP2 hybrid orbitals overlap with the SP2 hybrid orbitals of adjacent carbon atoms to form six C-C sigma bonds in the hexagonal plane. Each carbon atom's remaining SP2 hybrid orbital overlaps with a hydrogen atom's s-orbital to form six C-H sigma bonds. Each carbon atom now has one hybridised p-orbital perpendicular to the ring plane. C-atoms' unhybridized p-orbitals are close enough to form a bond via lateral overlap.
2. N – pentane has higher boiling point than neopentane but the melting point of neopentane is higher than that of n – pentane.
The surface area and vander wall forces of attraction in neopentane are much weaker than in n-pentane due to the presence of branches. As a result, the boiling point of neopentane is lower than that of n-pentane. The melting point is determined by the arrangement of molecules in the crystal lattice. Because neopentane is more symmetrical than n-pentane, it packs much more tightly in the crystal lattice than n-pentane and thus has a much higher m.p than n-pentane.
Conclusion
Deactivating groups are substituents that decrease the rate of a reaction.
The groups which direct the incoming group to meta position are called meta-directing groups.
Meta acts as a Deactivating Group because they don’t tend to donate electrons.
Benzene is the most fundamental organic, aromatic hydrocarbon.
Benzene is also cyclohexatriene.
Multiple Choice Questions
1. The bond length of c-c bond in benzene is
(a) 1.45 A
(b) 1.38 A
(c) 1.33 A
(d) 1.23 A
Answer: (b)
2. Which of the following reagents does not react with benzene?
(a) Concentrated H2SO4
(b) HNO3 / H2SO4
(c) Iodoethane in the presence of iron
(d) Sodium hydroxide solution
Answer: (d)
3. CHO group on benzene nucleus
(a) Activates ring
(b) Deactivates ring
(c) Does not affect the ring
(d) None of the above
Answer: (b)
4. Identify deactivating and meta directing groups from the following
(a) -CHO
(b) -NH2
(c) -OH
(d) -OCH3
Answer: (a)
FAQs on Deactivating and Meta Directing Groups Explained
1. What does it mean for a group to be 'deactivating' in electrophilic aromatic substitution?
A deactivating group is a substituent attached to a benzene ring that makes the ring less reactive towards an incoming electrophile compared to pure benzene. These groups typically pull electron density away from the ring through effects like induction or resonance, making it less attractive to an electron-seeking electrophile.
2. How can I quickly identify if a group attached to a benzene ring is deactivating?
A simple way to identify a deactivating group is to look at the atom directly bonded to the ring. If this atom has a positive or partial positive charge, or is part of a multiple bond with a more electronegative atom (like C=O or N=O), it's usually a deactivating group. For example, groups like -NO₂, -CN, and -COOH are strong deactivators.
3. What are some common examples of groups that are both deactivating and meta-directing?
Many common deactivating groups are also meta-directing. Here are a few key examples you'll encounter in your chemistry syllabus:
- Nitro group (-NO₂)
- Cyano group (-CN)
- Carbonyl groups, found in aldehydes (-CHO), ketones (-COR), and carboxylic acids (-COOH)
- Sulfonic acid group (-SO₃H)
4. Why do most deactivating groups direct an incoming electrophile to the meta position?
This is due to the stability of the intermediate carbocation (arenium ion) formed during the reaction. When an electrophile attacks at the ortho or para positions, one of the resonance structures places a positive charge directly next to the electron-withdrawing group. This is a very unstable arrangement. An attack at the meta position avoids this highly unstable structure, making it the most favourable and fastest pathway for the reaction to proceed.
5. What is the difference between the inductive effect and resonance effect in making a group deactivating?
The inductive effect is the withdrawal of electron density through the single (sigma) bonds, caused by an atom's high electronegativity. The resonance effect (or mesomeric effect) involves pulling electron density out of the ring's pi system through delocalisation across multiple bonds. While both contribute, a strong deactivating resonance effect is a clear indicator that a group will also be meta-directing.
6. Are all deactivating groups also meta-directing? What about halogens?
No, and halogens are the most important exception. Halogens (-F, -Cl, -Br, -I) are deactivating because their high electronegativity pulls electron density from the ring through the inductive effect. However, they are ortho-para directing because their lone pairs can donate electron density back to the ring through the resonance effect. This resonance donation specifically stabilises the intermediates for ortho and para attack, making those positions more favourable despite the ring being less reactive overall.
























