




How Does Magnesium Bicarbonate Form and Why Is It Important?
A salt is formed, along with water, as a product of the neutralisation reaction between an acid and a base. Some salts are acidic, some are basic while some are neutral. Acidic salts are formed after a reaction between a strong acid and a weak base, a basic salt is formed after the neutralisation of a weak acid by a strong base and a neutral salt is formed by the action of a strong acid on a strong base. $Mg(HCO_{3})_{2}$ is also a salt formed by magnesium hydroxide and carbonic acid by neutralisation reaction. It is a basic salt since it is formed by the hydroxide of an alkaline earth metal and a weak acid.
Preparation of Magnesium Bicarbonate
There are multiple methods of preparation of $Mg(HCO_3)_2$ . Two of them are:
Neutralisation reaction between magnesium hydroxide $(Mg(OH)_2)$ and carbonic acid $(H_2CO_3)$ . The reaction of the same is as follows:
$Mg(OH)_2 \ + \ 2H_2CO_3 \xrightarrow[Reaction]{Neutralisation} \ Mg(HCO_3)_2 \ + \ 2H_{2}O$
Double displacement reaction between sodium bicarbonate $(NaHCO_3)$ and magnesium acetate $(Mg(C_2O_4))$ where sodium and magnesium salts exchange their respective anions. The reaction of the same is as follows:
$Mg(C_2O_4) \ + \ 2NaHCO_3 \xrightarrow[Reaction]{Double \ displacement} \ Mg(HCO_3)_2 \ + \ Na_2(C_2O_4)$
Structure of Magnesium Bicarbonate
Magnesium $(1s^22s^22p^63s^2)$ loses two of its electrons to become dipositive $Mg^{2+} \ ((1s^22s^22p^6)$ cation. It acts as a centre of positive charge. Carbonic acid loses one proton $(H^+)$ to become a uninegative anion $HCO^-_3$. It acts as a centre of negative charge. The bonding between hydrogen, carbon, and oxygen here are covalent, i.e., due to the overlapping of partially filled orbitals. Together, both these centres of opposite charges are attracted towards each other due to electrostatic force leading to the formation of an ionic bond.
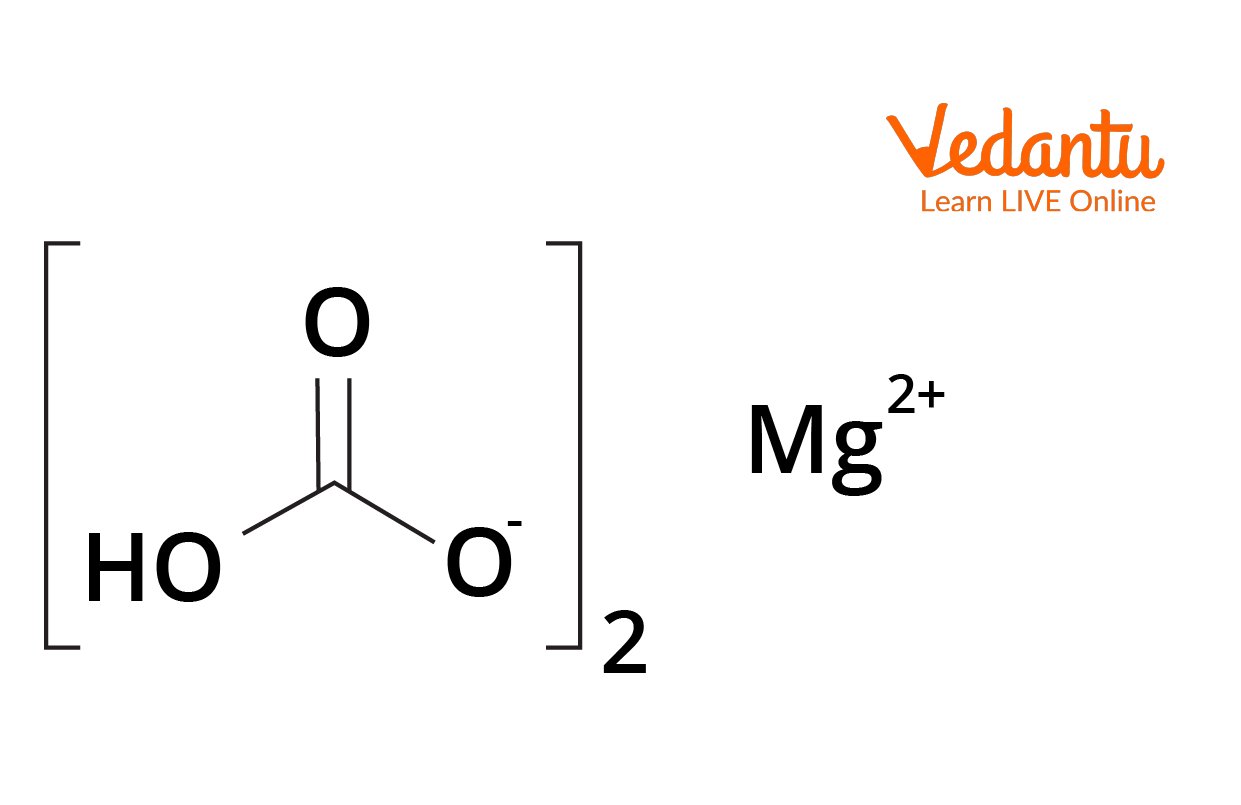
Structure of Magnesium Bicarbonate
Characteristics of Salts
There are infinite pairs of acids and bases possible. Due to this, there is a vast variety in types of salt. Each of these shows a difference in their physical and chemical properties. Some of these are crystalline while others may be amorphous, some may be coloured while others may be white, some might have a pungent odour while others may be odourless and so on.
State of Existence
Only alkali metal bicarbonates, except $LiHCO_3$, exist in solid state. Other than alkali metals, only $NH_4HCO_3$ exists in solid state. No other element in the periodic table forms stable bicarbonate in solid state. Therefore, magnesium bicarbonate $(MgHCO_3)$ does not exist in the solid state.
pH of Salt
Magnesium bicarbonate $(MgHCO_3)$ is a product of the neutralisation reaction between magnesium hydroxide $(Mg(OH)_2)$ and carbonic acid $(H_2CO_3)$. Therefore, it is a basic salt of pH greater than $7 \ (8.3)$
Physical Properties of Magnesium Bicarbonate
Those characteristics of matter which are not concerned with change in their chemical composition are called physical properties. Examples include boiling point, solubility, melting point, density, electrical connectivity, etc.
1. Boiling Point
The temperature at which a liquid turns to vapour is known as its boiling point. Magnesium bicarbonate exists in solution state at room temperature. Its boiling point is about $333.6^o$ C at $760$ mm of Hg.
2. Solubility
The maximum amount of solute that will dissolve in the solvent at a particular temperature is known as the solubility of the solute. It is dependent on two opposing factors - lattice energy and hydration energy. For a substance to be soluble, its lattice energy should be lower than its hydration energy. For $Mg(HCO_3)_2$ , the solubility in water is $5.7 \ gm/100 \ mL$ at $20^o$ C.
Uses of Magnesium Bicarbonate
It is useful in maintaining proper muscular and nervous functions.
It helps in the growth of bones.
Magnesium is an important mineral of the body that helps in metabolic functions and $Mg(HCO_3)_2$ is a good source of it.
It supports the energy metabolism.
Due to its basic nature, it can potentially be used as an antacid to relieve heartburn, indigestion and upset stomach.
It also helps maintain the ionic balance in the body.
Chemical Properties of Magnesium Bicarbonate
1. Thermal Decomposition
Any acid including oxygen in its structure is called an oxyacid and the salt of any such acid is called an oxysalt. The oxysalt of alkaline earth elements aren’t thermally the most stable and thus, they decompose on heating.
Since $Mg(HCO_3)_2$ is a salt of oxyacid $H_2CO_3$, it is an oxysalt. The thermal decomposition of the same takes place on heating and the reaction followed is $Mg(HCO_3)_2 \ \overset{\bigtriangleup }{\rightarrow} \ MgCO_3 \ + \ H_2O \ + \ CO_2 \overset{\bigtriangleup }{\rightarrow} \ MgO \ + \ CO_2$
2. Association with Hardness of water
The water unable to cause lathering of soap is called hard water. The cause of hardness of water are soluble salts of magnesium and calcium which precipitate the hydrocarbon chain of soap thus preventing lathering. Therefore, the solubility of $Mg(HCO_3)_2$ is the main cause of hardening of water. The reaction of soap ($RCOONa$) with Magnesium bicarbonate ($Mg(HCO_3)_2$) can be understood as $ RCOONa \ + \ Mg(HCO_3)_2 \rightarrow \ Mg(RCOO)_2 \ + \ 2NaHCO_3$
Summary
The cause of the temporary hardness of water, which is magnesium bicarbonate, is a basic salt of pH 8.3. It isn’t stable in solid state, therefore, it is always present as a solution. Thermally unstable, it can easily be decomposed to $MgCO_3$ on heating. Production of magnesium bicarbonate is performed either by double displacement reaction or by acid-base neutralisation reaction. It has multiple uses along with being used as a common antacid.
FAQs on Magnesium Bicarbonate: Key Concepts, Properties & Uses
1. What would happen if magnesium bicarbonate was to react with nitric acid?
Magnesium bicarbonate is a salt of a carbonic acid, which is a weaker acid than nitric acid. By nature, any weak acid or base is replaced in its salt by a stronger acid or base. The reaction thus occurring would be $ Mg(HCO_3)_3 \ + \ 2HNO_3 \ \rightarrow \ Mg(NO_3)_2 \ + \ 2CO_2 + 2H_2O$.
2. How can hardness due to $Mg(HCO_3)_2$ be removed?
Hardness of water due to bicarbonate salts can be removed by boiling and therefore, it is called temporary hardness of water. Another way to remove temporary hardness is by using a calculated amount of $CaO$ or quick lime. This is known as Clark’s method. The reaction occurs according to the equation $CaO + H_2O \rightarrow 2Ca \left ( OH \right )_2$ which further reacts with Magnesium bicarbonate as $Mg\left ( HCO_3 \right )_2+ 2Ca\left ( OH \right )_2 \rightarrow 2CaCO_3 \downarrow + Mg\left ( OH \right )_2 \downarrow + 2H_2O$.
But, if excess $CaO$ is added, water again becomes hard since the reaction $Ca(OH)_2 + H_2O + CO_2 \rightarrow Ca(HCO_3)_2$ is followed.
3. Why does the thermal stability of oxysalts like carbonates, bicarbonates, sulphates, nitrates, etc. increase down the group?
On moving down the group, the size of the cation increases. Therefore, the tendency of the cation to polarise anion of oxysalts decreases. Also, the ability of cations to form stable oxide by decomposition of a compound is decreased down the group. Thus, the stability of oxysalts increases down the group.
























