




How to Identify and Classify Molecules in Chemical Compounds
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds. Molecules are the smallest particles of a substance that have all of the physical and chemical properties of that substance. Biological molecules such as protein and DNA are made up of many thousands of atoms. A compound is a molecule made of atoms from different chemical elements. Compounds can be classified into two types, i.e., molecular compounds and ionic compounds. They can be broken down chemically only. Compounds contain a fixed ratio of atoms, held together by chemical bonds. Compounds are homogeneous in nature and cannot be separated physically.
What are Molecules of Compounds?
A combination of two or more atoms of different types is called a molecule of the compound. This means molecules of compounds have atoms of two or more different chemical elements, for example, methane, water, carbon dioxide, ammonia etc. We can further classify them on the basis of the number of atoms present in the molecule. The chemical bonding between the atoms can be either a covalent bond or an ionic bond. Ionic bonds always form between a molecule which has cations (positive ions) and anions (negative ions). Therefore, ionic compound always forms between two different chemical elements. Covalent bonds are formed by equal sharing of electrons between two atoms.
What are the Types of Elements and Compounds?
Elements are divided into three types:
Non-metals and
Metals: Substances having characteristics properties like malleability, ductility, sonority, electrical and thermal conductivity, lustre and solidness are called metals. Metals have high melting points. Most pure metals come from the earth’s crust. They are found in ores, which is a solid material. For example, zinc, iron, copper, aluminium, lead, chromium, cadmium, nickel, tin, zinc etc.
Non-metals: Non-metals are substances that do not conduct heat and electricity and are neither malleable nor ductile. For example, carbon, sulphur, phosphorus, silicon, oxygen etc.
Metalloid: A metalloid is a type of chemical element which has properties of both metals and nonmetals. The properties of the metalloids lie in between the metals and non-metals. For example, arsenic, silicon, boron etc.
Compounds are classified into two types:
Molecular compounds
Ionic compound.
Molecular Compounds: Molecules can be defined as the compound which can be formed by the combination of the same atom or different atoms. The atoms are joined to give a definite shape which is defined by the angles between the bonds and by the bond lengths. For example, carbon dioxide, water, ammonia etc.
Ionic Compound: It is made up of positive ions and negative ions. They break completely into ions when dissolved in water. For example, sodium chloride (NaCl), potassium chloride (KCl), copper sulphate (CuSO4) etc.
Examples of Molecules of Compounds
Some examples of molecules of a compound are given below:
H2O, NH3, CH4, CO2 are molecules of compounds with covalent bonding.
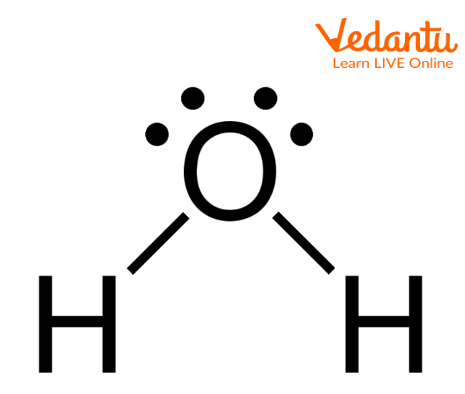
Water Molecule
The above image shows the structure of molecules of water in which two hydrogen atoms are covalently bonded with oxygen atoms and two lone pairs are present on oxygen atoms. The geometry of the water molecule is tetrahedral and the shape is bent.
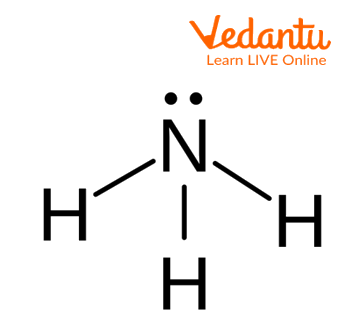
Ammonia Molecule
The above image shows the structure of molecules of ammonia in which three hydrogen atoms are covalently bonded with one nitrogen atom and one lone pair of electrons is present on the nitrogen atom. The geometry of the ammonia molecule is tetrahedral and the shape is pyramidal.
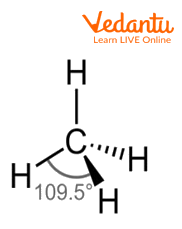
Methane Molecule
The above image shows the structure of molecules of methane in which four hydrogen atoms are attached to one carbon atom and form tetrahedral geometry.

Carbon Dioxide
The above image shows the structure of carbon dioxide in which two oxygens are bonded with one carbon atom by a double bond and form linear geometry.
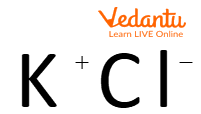
Ionic Compounds
The above image shows the ionic interaction of potassium chloride.
Key Features
Molecules of compounds are the combination of two or more atoms of different types.
Metals can conduct electricity but non-metals cannot conduct electricity.
A metalloid has properties between metals and nonmetals.
The nature of compounds is homogenous.
FAQs on Molecules of Compounds: Definition, Examples & Uses
1. What is the definition of a molecule of a compound?
A molecule of a compound is the smallest electrically neutral unit of a substance formed when atoms of two or more different elements are chemically joined together in a fixed, definite ratio. For example, a molecule of water (H₂O) always consists of two hydrogen atoms bonded to one oxygen atom. This smallest particle retains all the chemical properties of that compound.
2. What type of chemical bond is characteristic of molecular compounds?
The characteristic bond in a molecular compound is the covalent bond. This type of bond is formed when atoms share electrons with each other to achieve stability. This sharing of electrons holds the atoms together to form a discrete molecule, unlike ionic compounds where electrons are completely transferred from one atom to another.
3. How are molecules of compounds formed?
Molecules of compounds are formed when atoms of different elements approach each other and their electron clouds interact. If the overall energy of the system is lowered by sharing electrons to form covalent bonds, the atoms will chemically bond together. This process results in a stable, new substance with properties distinct from its constituent elements.
4. What are some common examples of molecules of compounds used in daily life?
Many substances we use daily are molecules of compounds. Here are a few examples:
- Water (H₂O): Essential for life, used for drinking, cooking, and cleaning.
- Carbon Dioxide (CO₂): Used by plants for photosynthesis and in carbonated drinks.
- Methane (CH₄): The main component of natural gas, used as fuel for cooking and heating.
- Ammonia (NH₃): Commonly found in household cleaners and used to produce fertilisers.
- Sucrose (C₁₂H₂₂O₁₁): Commonly known as table sugar, used as a sweetener.
5. What is the key difference between a molecule of an element and a molecule of a compound?
The key difference lies in the types of atoms they contain. A molecule of an element consists of one or more atoms of the same element (e.g., Oxygen gas, O₂; Ozone, O₃). In contrast, a molecule of a compound consists of atoms of different elements chemically bonded together (e.g., Water, H₂O; Carbon Dioxide, CO₂).
6. Why is table salt (NaCl) typically classified as an ionic compound, not a molecular compound?
Table salt (Sodium Chloride, NaCl) is not a molecular compound because it is formed by the transfer of electrons, not sharing. A sodium atom donates an electron to a chlorine atom, creating charged ions (Na⁺ and Cl⁻). These ions are held together by strong electrostatic forces in a large, ordered crystal lattice, rather than forming discrete, individual NaCl molecules.
7. Why is the fixed ratio of atoms in a molecule of a compound so important?
The fixed ratio of atoms, as described by the Law of Definite Proportions, is crucial because it defines the identity and properties of a compound. If the ratio changes, the substance changes entirely. For example, water (H₂O) has a 2:1 ratio of hydrogen to oxygen and is essential for life. Hydrogen peroxide (H₂O₂), with a 2:2 (or 1:1) ratio, is a completely different compound used as a disinfectant.
8. How can a molecule of a compound like water be broken down?
A molecule of a compound cannot be broken down by physical means (like boiling or filtering). It can only be broken down into its constituent elements through a chemical reaction. For instance, a molecule of water (H₂O) can be broken down into hydrogen gas (H₂) and oxygen gas (O₂) by passing an electric current through it, a process known as electrolysis.
























