




What Is Moscovium? Element Facts, Discovery, and Exam Tips
It is a transactinide element in the p-block of the periodic table. Although it has not been proved that it behaves as a heavier homologue of the pnictogen bismuth, it is a member of the 7th period and is listed as the heaviest pnictogen in group 15. Moscovium is predicted to be a post-transition metal and to have certain characteristics with its lighter homologues, nitrogen, phosphorus, arsenic, antimony, and bismuth, while it should also exhibit a number of significant deviations from them. Moscovium in particular ought to resemble thallium quite a bit because both elements have a single electron that is only loosely bound outside of a quasi-closed shell.
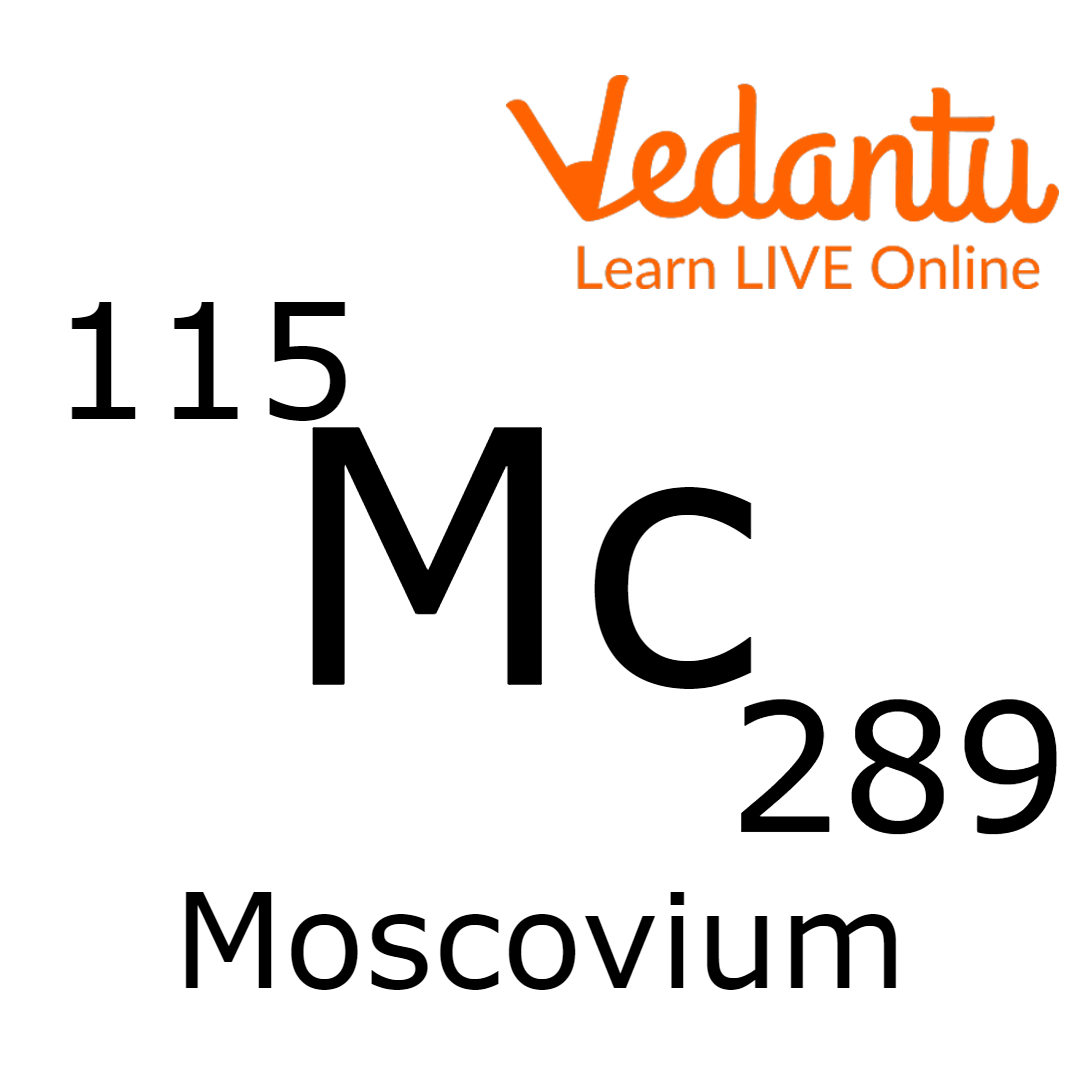
Moscovium
Discovery of Element 115
Element 115 was found in 2003, and its discovery was declared on February 2, 2004. The Joint Institute for Nuclear Research in Dubna, Russia, and the Lawrence Livermore National Laboratory in the United States jointly developed and released it.
Scientists blasted americium atoms, element 95, with calcium ions, with an atomic mass of 20, to create this new element. Within a particle accelerator, heavy atoms are bombarded. Light atoms, ions, or subatomic particles like neutrons are frequently used as hitting particles. This time, calcium ions were involved.
The experiment of blasting americium with calcium ions was replicated by researchers at the Helmholtz research facility in Darmstadt, Germany, and they were able to confirm the synthesis of element 115.
The International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics (IUPAC and IUPAP) confirmed the synthesis of the new element after it had been created.
Isotopes of Moscovium
Moscovium has four known isotopes, but the two heaviest isotopes are 289 (289Mc ) and 290 (290Mc).
These isotopes undergo alpha decay to create nihonium, element 113, daughter nuclei.
The element is classified as a metal and is known to exist in nature as a solid at normal temperature.
Out of the four isotopes of ununpentium identified so far,the most stable isotope is 289 Uup, which has a half-life of roughly 220 milliseconds.
Ununpentium is thought to be the third chemical element in the 7p block and is the heaviest element in group 15 of the periodic table.
Physical Properties
According to scientific expectations, the element will be solid at room temperature. The approximate melting and boiling points of moscovium are 670 K and 1400 K, respectively.
As far as appearance is concerned, it will be a silvery gray tint.
Moscovium is expected to have a melting point similar to nihonium at about 400°C.
Similar to nihonium, it is projected that the boiling point of moscovium will be around 1100°C.
Moscovium has an estimated density of 13.5 g/cc.
The metallic bond strength of moscovium is comparable to that of nihonium.
The half-life of the moscovium isotope with the longest half-life is 0.65 seconds.
Chemical Properties
Only the theoretical simulations provide all of the information regarding the properties of moscovium though its chemical properties haven't been determined in a clear-cut way yet.
Moscovium can be in the following likely oxidation states: +1, +3, and +5, with +3 being the most prevalent.
Compounds made of moscovium are predicted to favor the '+1' oxidation state.
Compounds containing moscovium are not anticipated to have an oxidation state of "+5"; rather, such compounds are assumed to be unattainable in nature.
Uses of Moscovium
Since there are so few moscovium atoms in existence, they are primarily used for research.
Metal moscovium is also produced using it.
It plays no biological function. However, the metal is viewed as dangerous due to claims that it is highly radioactive.
Interesting Facts About Moscovium
The element had been given the placeholder name ununpentium, which is Latin for "one-one-five.
" The name "moscovium" for element 115 was accepted by the IUPAC (International Union of Pure and Applied Chemistry) in November 2016.
In 2016, Ununpentum adopted the moniker Moscovium. The location of the experiments to create this element is recognized by the name "moscovium."
Moscovium cannot be created naturally in the Earth's crust; instead, it must be created artificially in particle accelerators.
All the isotopes of moscovium that have been characterized thus far have been created synthetically, and it cannot even be produced in a nuclear reactor.
Summary
Moscovium, which has the atomic number 115 and the chemical symbol Uup, is a radioactive element that was synthesized and is extremely heavy. It was found by ionizing calcium-48 to attack the atoms of the element Americium-243. The substance is thought to exist naturally as a solid at room temperature and is categorized as a metal. It immediately disintegrates into other substances like ununtrium. The substance is classified as a metal and is understood to be a solid in nature at room temperature. There are four known isotopes of ununpentium, the most stable of which is 289 Uup.
It is thought to be the third chemical element in the 7p block and is the heaviest element in group 15 of the periodic table.
FAQs on Moscovium: Properties, Definitions & Applications
1. What is Moscovium and what is its position in the periodic table?
Moscovium is a synthetic, superheavy chemical element with the atomic number 115 and the symbol Mc. It is located in Group 15 and Period 7 of the periodic table, placing it among the p-block elements. It is classified as the heaviest pnictogen, positioned directly below bismuth.
2. How is the element Moscovium synthesised in a laboratory?
Moscovium is not found in nature and is created artificially through nuclear bombardment. The synthesis involves bombarding a target of americium-243 (element 95) with a beam of high-energy calcium-48 (element 20) ions in a particle accelerator. This fusion of nuclei results in the formation of a few atoms of Moscovium, which are identified before they rapidly decay.
3. What are the predicted physical properties of Moscovium?
Since only a few atoms of Moscovium have ever been produced, its bulk physical properties have not been directly measured. However, based on its position in the periodic table, scientists predict the following:
Phase at STP: It is expected to be a solid at room temperature.
Melting Point: Predicted to be around 400 °C (670 K).
Boiling Point: Predicted to be around 1100 °C (1400 K).
Density: It is expected to be a very dense metal, with a predicted density of about 13.5 g/cm³.
4. What is the predicted electron configuration of Moscovium?
The predicted electron configuration for Moscovium (Mc) is [Rn] 5f¹⁴ 6d¹⁰ 7s² 7p³. This configuration places it in Group 15 of the periodic table, as it has five valence electrons in its outermost shell (7s² 7p³). This configuration is fundamental to predicting its chemical behaviour and oxidation states.
5. Is Moscovium radioactive, and what is its most stable isotope?
Yes, Moscovium is extremely radioactive. All its known isotopes are highly unstable due to their large, proton-heavy nuclei. The most stable known isotope is Moscovium-289 (Mc-289), which has a very short half-life of approximately 220 milliseconds. It typically decays into Nihonium-285 through alpha decay.
6. Why does Moscovium have no known commercial or biological applications?
Moscovium has no practical applications outside of scientific research primarily due to two factors: extreme instability and scarcity. Its isotopes decay in fractions of a second, making it impossible to accumulate enough material to use for any purpose. Furthermore, only a handful of atoms have ever been synthesised, restricting its use to the study of fundamental properties of matter.
7. How did Moscovium (Element 115) get its name?
Moscovium was officially named in 2016 by the International Union of Pure and Applied Chemistry (IUPAC). The name honours the Moscow Oblast in Russia, which is home to the Joint Institute for Nuclear Research (JINR) in Dubna. The team at JINR played a pivotal role in the discovery of this element through their collaborative experiments.
8. How do the predicted properties of Moscovium compare to other elements in Group 15, like Bismuth?
As the heaviest member of Group 15, Moscovium is expected to follow some periodic trends but also show significant differences due to relativistic effects. While it sits below Bismuth, its chemical properties might be distinct. For instance, while Bismuth commonly shows +3 and +5 oxidation states, Moscovium is predicted to have a stable +1 oxidation state in addition to a less stable +3 state. It is expected to be a volatile post-transition metal, similar to its lighter homologues.
9. What is the scientific importance of synthesising superheavy elements like Moscovium, even if they decay instantly?
The synthesis of superheavy elements like Moscovium is crucial for advancing our understanding of nuclear physics and chemistry. It allows scientists to:
Test the limits of the periodic table and the forces holding atomic nuclei together.
Explore the theoretical "island of stability," a predicted region where certain superheavy isotopes might have significantly longer half-lives.
Verify and refine models of nuclear structure and decay, which have broader implications for astrophysics and our understanding of the universe.
























