




Key Physical and Chemical Properties of Phenol
Any member of the family of organic compounds called phenols is distinguished by an attached hydroxyl (OH) group to a carbon atom of an aromatic ring. Monohydroxybenzene or phenol. The phenol formula is C6H5OH. Phenol is also known as benzenol or carbolic acid and it is the family's simplest member, and the term "phenol" serves as both the general name for the entire group and the specific name for it. While in this article we only discuss the phenol which has a benzene ring attached with a hydroxyl group.
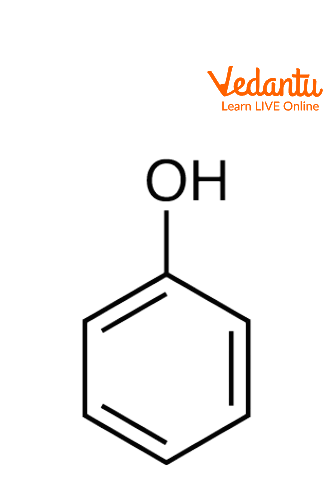
Structure of Phenol
General Properties of Phenol
The molecular formula of phenol is C2H5OH and molecular mass 94.113 g mol-1
It is a transparent crystalline solid with a sweet and tarry odour.
The density is 1.07 grams per centimetre cube, melting point is 40.5 °C and boiling point is 181.7 °C
Approximately 84.2 g of phenol dissolve in 1000 mL of water, making it a notable organic chemical that is water soluble (0.895 M). Sodium phenoxide, the sodium salt of phenol, is significantly more water-soluble.
Nomenclature of Phenols
The term "phenol" refers to organic substances that have at least one -OH group directly linked to the benzene ring. Phenols are divided into three categories: monohydric, dihydric, and trihydric, depending on how many hydroxyl groups are linked to the benzene ring.
Monohydric Phenols - Hydroxybenzene, often known as phenol, is the most basic compound in this group, while the others are referred to as substituted phenols. The term "cresols" refers to the three isomeric hydroxyl toluenes.
Dihydric Phenols - Catechol, resorcinol, and quinol are the three isomeric dihydroxy benzenes and are more commonly referred to by these names.
Trihydric Phenols - These are also referred to as trihydroxy phenols. Trihydric phenols are also known by the names pyrogallol, hydroxyquinol, and phloroglucinol.
Natural Occurrence of Phenols
Phenols are very commonly present in nature. Tyrosine is one of the standard amino acids found in most proteins. Epinephrine (adrenaline), is a stimulant hormone produced by the adrenal medulla. The essential oils of plants are utilised as flavours and fragrances. For instance, the flavour vanillin is extracted from vanilla beans. All these are examples of phenols. From the distillation of coal tar or crude petroleum, phenol, cresols (methylphenols), and other simple alkylated phenols can be produced.
Acidity of Phenols
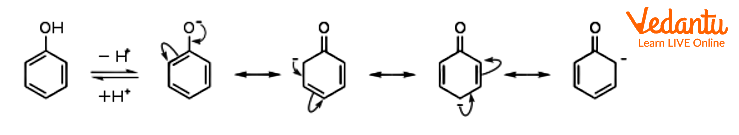
Resonance Structures of the Phenoxide Anion
Phenol is a weak acid. It is in equilibrium with the phenolate anion (also called phenoxide) C6H5O- in an aqueous solution in the pH range of approximately 8 to 12. Compared to aliphatic alcohols, phenol has a higher acidity. Resonance stabilisation of the phenoxide anion is thought to be the cause of the different pKa values. Through the pi system, the negative charge on oxygen is thereby delocalised onto the ortho and para carbon atoms.
Tautomerism in Phenols
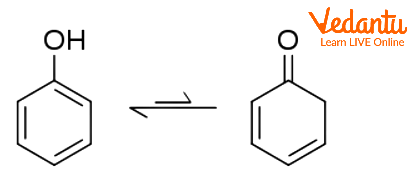
Phenol-cyclohexadienone Tautomerism
With its unstable keto tautomer cyclohexadienone, phenol exhibits keto-enol tautomerism; nonetheless, very little phenol is found in the keto form. Only one in ten trillion molecules are now in the keto form due to the equilibrium constant for enolization being approximately 10-13. The small amount of stabilisation gained by swapping a C=C bond for a C=O bond is more than compensated by the significant destabilisation brought on by the loss of aromaticity. As a result, phenol only really exists as enol.
Preparation of Phenol
Cumene Process
This is also known as the Hock process where Cumene (isopropylbenzene) is partially oxidised to cumene hydroperoxide in presence of air. Cumene hydroperoxide when treated with a dilute acid produces phenol and acetone. this process also yields significant amounts of acetone, a reaction byproduct. By using this technique, pure phenol can be obtained.
From Benzene Sulphonic Acid
${{C}_{6}}{{H}_{5}}S{{O}_{3}}Na\,+\,2NaOH\,\xrightarrow{573K}\,{{C}_{6}}{{H}_{5}}ONa+N{{a}_{2}}S{{O}_{3}}+{{H}_{2}}O$
$2{{C}_{6}}{{H}_{5}}ONa\,+\,2HCl\,\to \,2{{C}_{6}}{{H}_{5}}OH\,+\,2NaCl$
This is the first method used commercially to produce phenol. At 573 K, sodium benzene sulphonate and sodium hydroxide are fused to produce sodium phenoxide, which is then acidified to produce phenol.
From Diazonium Salts (Laboratory Method)
$Ar{{N}_{2}}^{+}{{X}^{-}}\,+\,{{H}_{2}}O\,\xrightarrow[{{H}_{2}}S{{O}_{4}}]{Warm}\,ArOH\,+\,{{N}_{2}}\,+\,HX$
${{C}_{6}}{{H}_{5}}{{N}_{2}}Cl\,+\,{{H}_{2}}O\,\xrightarrow[{{H}_{2}}S{{O}_{4}}]{\Delta }\,{{C}_{6}}{{H}_{5}}OH\,+\,HCl$
Phenol is produced when the diazonium salt solution is added to a boiling dilute sulphuric acid or when the diazonium salt solution is steam distilled.
Reactions of Phenol
A hydroxyl group is connected to an aromatic ring, which is highly ortho/para directing. Phenols have a high reactivity toward electrophilic aromatic substitution at their ortho and para carbons.
Aromatic ring reactions: Because of resonance, the hydroxyl group in phenol is ortho and para directing, increasing electron density at ortho and para positions. Thus, electrophilic substitution reactions occur in phenol.
Phenol Uses
Phenol is most frequently used to produce plastic precursors, accounting for two-thirds of its total production.
Phenol is also a useful precursor to a wide range of medications like aspirin, including several herbicides and pharmaceutical drugs.
In molecular biology, phenol is a component of the liquid-liquid phenol-chloroform extraction method used to extract nucleic acids from tissue or cell culture samples.
Many people use phenol as an antiseptic. Joseph Lister was the first to use it. For otology treatments, concentrated liquid phenol can be applied topically as a local anaesthetic.
Due to its low cost, phenol is used for numerous small-scale purposes. In order to remove epoxy, polyurethane, and other chemically resistant coatings, it is a component of industrial paint strippers used in the aviation sector.
The formulation of cosmetics has utilised phenol derivatives.
Toxicity of Phenols
Due to the protein-degenerating activity of the phenol, it has a corrosive effect on skin and mucosal membranes. Dermatitis or even second and third-degree burns may result from repeated or prolonged skin contact with phenol. Lung oedema could develop after inhaling phenol vapour. The phenol might affect the heart and central nervous system, causing dysrhythmia, seizures, and coma. The kidneys could also be impacted. The liver and kidneys may suffer negative consequences from prolonged or recurrent exposure to the drug.
Important Questions
Write a note on phenol solubility in water.
Ans: Approximately 84.2 g of the organic compound phenol can be dissolved in 1000 mL of water, making it a highly soluble substance (to form a 0.895 M solution). Phenol-to-water mass ratios of 2.6 and higher allow for homogenous phenol-water solutions. sodium phenoxide, the phenol sodium salt, is far more water soluble than phenol
Why is phenol considered to be an acid?
Ans: One may classify phenol as a weak acid. In aqueous solutions with a pH between 5 and 6, it is in equilibrium with the phenolate anion C6H5O-, commonly known as phenoxide. Since it contains an OH group and the aromatic ring resonance stabilises the phenoxide anion, phenol is more acidic than aliphatic compounds.
Summary
Phenol is an aromatic organic molecule having the chemical formula C6H5OH, also known as carbolic acid. It is a volatile white crystalline substance. A phenyl group and a hydroxyl group are joined to form the molecule. It is mildly acidic and should be handled carefully because it can result in chemical burns.
Originally extracted from coal tar, phenol is now produced in huge quantities (about 7 billion kg/year) from feedstocks supplied by petroleum. Due to its role as a precursor to numerous minerals and beneficial chemicals, it is a crucial industrial commodity. Plastics and related materials are largely created using it. The manufacture of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides like phenoxy herbicides, and several pharmaceutical medications all depend on phenol and its chemical derivatives.
Practice Questions
Which of these is not the name of phenol?
Carbolic acid
Hydroxybenzene
Phenic acid
Methylbenzene
Phenol is acidic due to the presence of which of these effects?
Resonance effect
Common ion effect
Steric effect
Solvation effect
Answers
(d)
(a)
FAQs on Phenol: Properties, Structure, Reactions, and Uses
1. What is Phenol, and what are its chemical formula and structure?
Phenol is an aromatic organic compound with the chemical formula C₆H₅OH. Its structure consists of a hydroxyl group (-OH) directly bonded to a carbon atom within a benzene ring. This direct attachment is key to its unique chemical properties, distinguishing it from aliphatic alcohols where the -OH group is attached to a non-aromatic carbon chain.
2. Why is Phenol considered acidic in nature?
Phenol is weakly acidic because it can donate a proton (H⁺) from its hydroxyl group to form a stable phenoxide ion (C₆H₅O⁻). The stability of this conjugate base is the primary reason for its acidity. The negative charge on the oxygen atom in the phenoxide ion is delocalised across the benzene ring through resonance. This charge distribution makes the phenoxide ion much more stable than an alkoxide ion (from an alcohol), thus facilitating the release of the proton.
3. What are the major industrial and commercial uses of Phenol?
Phenol is a versatile industrial chemical with numerous applications. Its primary uses include:
- Production of Polymers: It is a key monomer in the production of phenolic resins like Bakelite (used for electrical switches and adhesives) and polycarbonates.
- Chemical Synthesis: It serves as a precursor for synthesising a wide range of other chemicals, including caprolactam (for making nylon), bisphenol A (for epoxy resins), and pharmaceuticals like aspirin.
- Antiseptics and Disinfectants: Dilute solutions of phenol, once known as carbolic acid, are used in household cleaners and as disinfectants.
- Herbicides and Pesticides: Certain derivatives of phenol are used in the manufacturing of weed killers and other agrochemicals.
4. Why is Phenol significantly more acidic than ethanol?
Phenol is a stronger acid than ethanol due to the difference in the stability of their respective conjugate bases after donating a proton.
- In Phenol, the resulting phenoxide ion (C₆H₅O⁻) is stabilised by resonance, as the negative charge is delocalised over the entire benzene ring.
- In ethanol, the resulting ethoxide ion (CH₃CH₂O⁻) has its negative charge localised entirely on the oxygen atom. Furthermore, the ethyl group (-C₂H₅) is an electron-donating group, which intensifies the negative charge and destabilises the ion.
The greater stability of the phenoxide ion makes phenol more willing to release its proton, hence it is more acidic.
5. How does the hydroxyl group in Phenol influence its reactivity in electrophilic substitution reactions?
The hydroxyl (-OH) group is a powerful activating group for the benzene ring. The lone pair of electrons on the oxygen atom participates in resonance with the ring, which significantly increases the electron density at the ortho (2,6) and para (4) positions. This enrichment of electrons makes the ring highly attractive to electrophiles, causing electrophilic substitution reactions (like nitration, halogenation) to occur much more readily in phenol than in benzene. The -OH group thus directs incoming electrophiles primarily to the ortho and para positions.
6. What is the importance of the Reimer-Tiemann reaction for Phenol in organic synthesis?
The Reimer-Tiemann reaction is a crucial method in organic synthesis for the ortho-formylation of phenols. In this reaction, phenol is treated with chloroform (CHCl₃) in the presence of a strong base like sodium hydroxide (NaOH). The primary product is salicylaldehyde (2-hydroxybenzaldehyde). The importance of this reaction lies in its ability to introduce a carbonyl functional group (-CHO) onto the aromatic ring, which is a versatile starting material for synthesizing more complex molecules, including pharmaceuticals and fragrances.
7. What is the primary industrial method for the preparation of Phenol?
The most common and economically viable industrial method for producing phenol is the Cumene Process. The process involves three main steps:
- Step 1: Benzene is alkylated with propene in the presence of an acid catalyst to produce cumene (isopropylbenzene).
- Step 2: Cumene is then oxidized with air to form cumene hydroperoxide.
- Step 3: The cumene hydroperoxide is treated with a dilute acid (like H₂SO₄) to undergo cleavage, yielding Phenol and a valuable by-product, acetone.
8. What simple chemical test can be used to distinguish Phenol from an alcohol like ethanol?
A common and effective chemical test to distinguish phenol from alcohols is the Ferric Chloride (FeCl₃) Test. When a few drops of a neutral ferric chloride solution are added to an aqueous solution of phenol, a distinct violet or purple coloration appears. This is due to the formation of a coloured iron-phenol coordination complex. Aliphatic alcohols, like ethanol, do not have the specific electronic structure to form this complex and therefore do not give a positive result with this test.























