




Key Terms and Applications of UV Visible Spectroscopy
In Chemistry, as we deal with various compounds, the most common (as well as obvious) way we distinguish various compounds is by colour. The compounds with discernible colours reflect the light of a certain wavelength that our eyes capture. The light our eyes can capture represents a small width in the electromagnetic spectrum, which falls between 400-800 nm- this is called the visible spectrum. The lower wavelength band from 100-400nm represents the UV (ultraviolet) spectrum. Let us read ahead to know more about UV visible spectroscopy and its uses.
What is UV Visible Spectroscopy?
UV-visible spectroscopy is a technique that measures the amount of light absorbed by a chemical substance. It is absorption spectroscopy or reflectance spectroscopy technique within the ultraviolet and visible regions of the electromagnetic spectrum. When continuous radiation is passed through a compound a portion of that compound is absorbed by the compound. The residual radiation after passing through the compound yields a spectrum with gaps in it due to absorption by the compound, this spectrum is called the absorption spectrum.
Absorption of UV-Visible radiation results in the electronic transition of the compound, i.e., an electron in the ground state (occupied orbital) is promoted to the excited state (unoccupied orbital), and the amount of radiation absorbed corresponds to the energy difference between the ground state and the excited state.
UV Visible Spectroscopy Principle
UV-visible spectroscopy is a quantitative technique used in analytical chemistry to measure the amount of light absorbed by a substance. When light falls upon a substance it absorbs and reflects a certain amount of radiation. As the light passes through the sample, the amount of radiation absorbed by the substance is the difference between the incident radiation (Io) and the transmitted radiation (I). The amount of radiation absorbed is called absorbance (A) and transmittance (T), which is a fraction (I/ Io) indicating the amount of light that has passed through the sample.
Transmittance, T = I/ Io
Absorbance, A = log10(Io /I) = log10 (1/T) = - log10 (T)
According to Beer-Lambert’s Law, the absorbance of a solution (containing the compound) is directly proportional to the concentration of the absorbing species (the compound) and the path length. This translates to, as the number of molecules (concentration) capable of absorbing the radiation at a given wavelength increases, the extent of absorption is increased. Also, the efficiency of the molecule (recorded by its molar absorptivity) in absorbing the radiation contributes to greater absorption.
The formulation for Beer-Lambert’s law is given by
A= εcl
for a given wavelength
Where ε = molar absorptivity (also known as molar extinction coefficient)
c = molar concentration of the absorber (solute)
l = path length (length of the sample cell or cuvette; in cm)
The mathematical relation between absorbance and concentration established by the Beer-Lambert law allows direct measurement of the concentration of the absorber in a solution from absorbance for a fixed path length.
Instrumentation of UV Visible Spectroscopy
The instrumentation of UV-Visible spectroscopy is called a UV-Visible spectrophotometer. The spectrophotometer has a few key components, namely:
The light source emits broadband electromagnetic radiation across the UV-visible spectrum.
The dispersion device or monochromator - it’s a diffraction grating that separates the radiation into its component wavelength.
A sample area - the radiation interacts with the sample as it passes through or reflects off.
Detector - measures the reflected or transmitted radiation intensity.
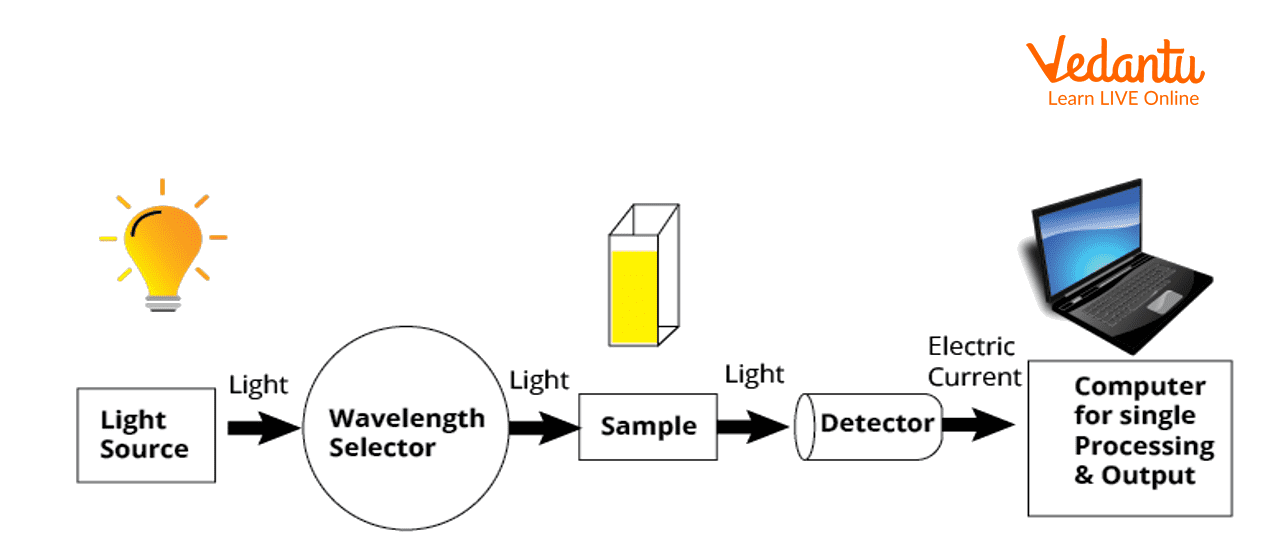
Representation of Working of the UV-Vis Spectrophotometry
The measurement is done by placing the sample in the sample compartment. Liquid samples are held in a rectangular holder made of glass, quartz or plastic, called a cuvette. Standard cuvettes have a 10mm path length and allow easy transmittance of ultraviolet radiation. The sample is placed in the path of the radiation as the beam from the monochromator passes through the sample to the detector.
The spectrophotometer compares the light intensity of the incident radiation (Io) before passing through the sample and the intensity of the transmitted radiation (I) that has passed through the sample and presents the absorbance value (A or Abs).
Application of UV Visible Spectroscopy
UV-Visible spectroscopy is widely used to determine the chemical and physical properties of substances. It can be used for:
Identification of molecules in a sample.
Determining the concentration of a compound in a sample.
Determine the purity or concentration of biological samples containing DNA or RNA.
It finds application in characterising the rate of a chemical reaction.
Advantages and Disadvantages of UV Visible Spectroscopy
Advantages
The method is non-destructive so that the sample can be reused.
The technique is fairly simple and can be used easily. No prior training is necessary.
Measurement can be done in a short span of time, helping easy integration into experiments.
Data analysis is simple and requires less processing.
Instrumentation is relatively inexpensive and can be procured easily by laboratories.
Disadvantages
Real instruments are not always perfect; hence stray light may interfere with the measurements.
Scattering of light due to bubbles or undissolved solid particles in the sample solution causes measurement error.
Beer-Lambert Law is only obeyed when a single absorbing species is present in the solution. A sample containing multiple absorbing species cannot be used to determine concentration using absorbance.
Improper orientation of the sample holder or misalignment can imbibe errors in the measurement.
Interesting Facts
Part of a molecule (usually an organic compound) that absorbs in the visible region gives colour to the compound and is called a chromophore.
Organic compounds with a high degree of conjugation absorb in the UV-Visible region.
Microspectrophotometry is the UV-visible spectroscopy of microscopic samples, where an optical microscope is fitted with UV-Visible optics.
Key Features
UV-Visible Spectroscopy is absorption spectroscopy.
In UV-Visible spectroscopy, compounds absorb light falling in the UV-Visible range of the electromagnetic spectrum.
UV-Visible Spectroscopy is measured by an instrument called UV-Visible Spectrophotometer.
The UV-Visible Spectrophotometer measures absorbance.
Beer-Lambert Law relates the concentration of the absorber with the absorbance of the solution.
FAQs on UV Visible Spectroscopy Explained: Concepts, Methods & Uses
1. What is UV-Visible Spectroscopy?
UV-Visible spectroscopy is an analytical technique used in chemistry to measure the amount of ultraviolet (UV) or visible light absorbed by a chemical substance. It works by passing a beam of light through a sample and detecting how much light is absorbed at each wavelength. This absorption corresponds to the electronic transitions of electrons from a ground state to a higher energy excited state within the molecule.
2. What is the fundamental principle of UV-Visible Spectroscopy?
The fundamental principle is based on the Beer-Lambert Law. This law states that the absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of the light through the solution. Essentially, the more molecules there are in the light's path, the more light will be absorbed. The relationship is described by the equation A = εcl, where A is absorbance, ε is the molar absorptivity, c is the concentration, and l is the path length.
3. What are the main components of a UV-Visible Spectrophotometer?
A UV-Visible spectrophotometer, the instrument used for this technique, consists of several key components working in sequence:
- Light Source: Emits a broad spectrum of light covering both UV and visible ranges.
- Monochromator: A device, often a diffraction grating, that separates the broadband light into its individual wavelengths, allowing only a specific wavelength to pass through.
- Sample Area: Holds the sample, typically in a transparent container called a cuvette, in the path of the light beam.
- Detector: Measures the intensity of the light that has passed through the sample.
- Readout Device: Displays the data, usually as an absorbance value.
4. What are some common real-world applications of UV-Visible Spectroscopy?
UV-Visible spectroscopy is a versatile technique with many practical applications, including:
- Quantitative Analysis: Determining the concentration of a known substance in a solution, such as the amount of a metal ion in a water sample.
- Purity Assessment: Checking the purity of organic compounds or biological samples, like determining the concentration and purity of DNA or RNA.
- Chemical Identification: Aiding in the identification of compounds by comparing their absorption spectra to known standards, particularly for molecules with chromophores.
- Reaction Kinetics: Monitoring the rate of a chemical reaction by observing the change in concentration of a reactant or product over time.
5. What is the difference between the Ultraviolet (UV) and Visible regions used in this technique?
The difference lies in their respective wavelength ranges on the electromagnetic spectrum. The Ultraviolet (UV) region covers wavelengths from approximately 100 to 400 nanometers (nm), which are shorter and higher in energy than visible light. The Visible region covers wavelengths from about 400 to 800 nm, which is the range of light the human eye can perceive as colour.
6. How is the concentration of a substance determined using the Beer-Lambert Law in UV-Vis analysis?
To determine a substance's concentration, a series of standard solutions with known concentrations are prepared and their absorbance is measured at a specific wavelength (λ_max). A calibration curve is plotted with absorbance versus concentration, which should yield a straight line. The absorbance of the unknown sample is then measured under the same conditions. By finding where this absorbance value falls on the calibration curve, the corresponding concentration of the unknown sample can be accurately determined.
7. Why do some chemical compounds appear coloured while others are colourless?
A compound appears coloured if it absorbs light within the visible spectrum (400-800 nm). The colour we perceive is the complement of the colour that is absorbed. This absorption is caused by molecular structures called chromophores, which often have a high degree of electron conjugation (alternating single and multiple bonds). Compounds that are colourless absorb light only in the higher-energy UV region, which is invisible to the human eye.
8. What are the key advantages and limitations of using UV-Visible Spectroscopy?
UV-Visible Spectroscopy is widely used due to its many advantages, but it also has some limitations.
Advantages:
- It is a simple, rapid, and easy-to-use technique.
- The method is non-destructive, meaning the sample can be recovered and used for further analysis.
- It is highly effective for the quantitative analysis of compounds containing chromophores.
- The instrumentation is relatively affordable and common in labs.
- The technique is less effective for samples with multiple species that absorb at similar wavelengths.
- It provides limited information about the detailed molecular structure.
- Light scattering from suspended particles or bubbles in the sample can cause significant measurement errors.
9. What is the specific role of a chromophore in UV-Visible absorption?
A chromophore is the specific part of a molecule that is responsible for absorbing light in the UV or visible regions. It is typically a group of atoms containing multiple bonds (like C=C, C=O, N=N) or atoms with non-bonding electrons. When light of the correct energy strikes the chromophore, it excites an electron from a lower-energy molecular orbital to a higher-energy one. The presence and structure of the chromophore determine the specific wavelength of light a molecule will absorb, and thus its potential colour.
10. Can UV-Visible Spectroscopy identify a completely unknown substance? Explain why or why not.
Generally, no. UV-Visible Spectroscopy is not sufficient on its own to identify a completely unknown substance. The absorption spectrum it produces is often broad and lacks the highly specific fingerprint details needed for definitive structural identification. While the spectrum can suggest the presence of certain types of chromophores and the extent of conjugation, many different compounds can have very similar spectra. It is best used to confirm the identity of a suspected compound by matching its spectrum to a known standard or for quantitative analysis of a known substance.
























