




Overview of Air Pressure
Everything in the universe is made up of matter, and we know that all matter has weight. Air is also made up of matter. Does that mean air also has weight? If yes, then why do we not feel it? How does air not crush us?
Air surrounds us all the time, no matter where we go or what we do. Even though we can’t feel the air around us, it is always there, and it exerts a force on us. This force that the air exerts on everything is known as air pressure. The earth is wrapped in a thick blanket of gases known as the atmosphere, which exerts air pressure. In this article, we will answer questions about what air pressure is, what the air pressure at the water level is, how is air pressure underwater, etc.
What is the Air Pressure on the Water Level?
Since the atmosphere is wrapped around the earth and the air molecules in it weigh down on the surface in the form of air pressure. The pressure is highest when you are closer to the surface of the earth. As you go higher up towards the atmosphere, it reduces.
The air pressure exerted by the atmosphere at the water level (also known as sea level) is 14.7 psi, where psi refers to Pounds per Square Inch. Thus, for every square inch on the surface of the earth, the atmosphere presses down 14.7 pounds. This pressure is a direct result of the earth's gravitational force. A very simple explanation is that gravity pulls the air molecules towards the surface of the earth, causing them to weigh down on the surface.
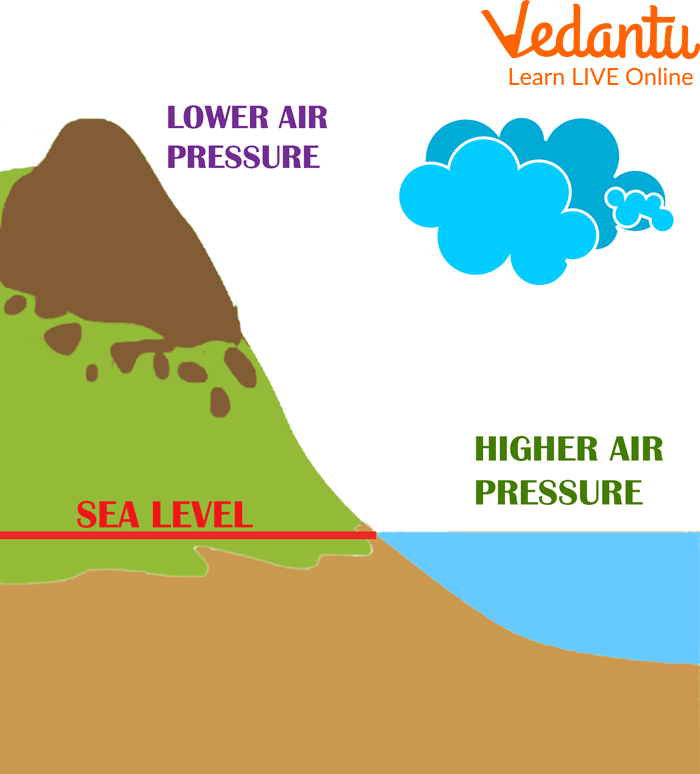
Air Pressure Increases as one Moves Upward
Air Pressure in Water
What happens to air pressure if you are submerged in water? Does air pressure on water have no effect? Or does the air pressure add to the weight of the water molecules?
One unit of atmospheric pressure (14.7psi) is the amount of weight a section of water 10.3m in depth exerts on a submerged body. But the atmosphere also exerts pressure on every square inch of the surface of the earth, including water, so how much pressure is exerted on a submerged body?
A body submerged in water at 10.3m experiences 2 atmospheres. (1 atmosphere of water plus 1 atmosphere of air). In water, the atmospheric pressure increases the deeper underwater the body goes. This is why submarines have special pressurised structures that allow them to float deep underwater. It is also why deep sea divers need special suits, or ship-wreck survivors need to be put in pressurised chambers after being rescued.

Submarines need to have specialised Structures to Survive high Pressure
Uses of Air Pressure
Air pressure has a wide variety of functions. Some of the functions of air pressure are -
The simplest use of air pressure is in aeroplanes or submarines, as they both exist in areas of extreme pressure conditions.
It is used in weather prediction.
Air pressure works in inflating tyres as well as when drinking drinks from straws.
It is also used when playing wind-based instruments.
Interesting Facts About Air Pressure
The instrument used to measure atmospheric pressure is known as a barometer.
Air pressure readings can also be used to predict natural disasters such as tornadoes.
One unit of air pressure is 14.7 psi is also called 1 atm (1 atmosphere).
Water in air pressure exists as a liquid state unless there are changes in temperature. At 100⁰C, it boils to form steam (gas), and at 0⁰C, it freezes to form ice (solid).
If pressure is affected by temperature, gravity, and altitude.
Atmospheric pressure is usually highest when the air is warm and dry.
Summary
Air molecules are all around us, and they all have weight. The atmosphere, which is made up of air, wraps around the earth and weighs down on the surface. In this article, we learned that air pressure refers to this pressure exerted by the atmosphere on the surface of the earth. We also discovered several interesting facts about air pressure, like air pressure on the surface level is 14.7 psi, and it decreases as you go up in altitude.
We also learned that when in water, the weight of the water molecules adds to the air pressure exerted by the atmosphere, so the deeper you go underwater, the more the pressure increases. Air pressure also has multiple uses, such as in making airplanes and submarines, as well as in simple things such as inflating tires and using straws.
FAQs on Air Pressure on Water
1. What exactly is air pressure?
Air is all around us, and this air has weight. Air pressure is the force of this air pushing down on every surface, including our bodies and the ground. Think of it like being at the bottom of a deep ocean of air; the weight of all that air above creates pressure.
2. How does air pressure affect the water in a glass or a lake?
Air pressure constantly pushes down on the surface of all water. Usually, this push is balanced, so we don't notice it. However, this pressure is strong enough to hold water in an upside-down glass if covered with cardboard. It also affects the boiling point of water; on high mountains with less pressure, water boils at a lower temperature.
3. Why don't we feel the heavy weight of air pushing on us all the time?
This is a great question! We are not crushed by air pressure because the pressure inside our bodies pushes outwards with an equal force. The pressure from the inside balances the pressure from the outside, so we don't feel it at all.
4. How does using a straw to drink juice show the power of air pressure?
When you suck on a straw, you remove the air inside it, creating an area of low pressure. The normal air pressure outside the straw is now much higher. This higher pressure pushes down on the surface of the juice, forcing it up through the straw and into your mouth.
5. Why does air pressure get lower when you go up a high mountain?
As you climb higher, the amount of air above you decreases. Since there is less air stacked on top of you, its weight is less, which means the air pressure is lower. This is also why it can be harder to breathe on very high mountains, as the air is less dense.
6. How can I do a simple experiment at home to see air pressure on water?
You can easily see air pressure at work with this fun experiment:
- Fill a glass to the very top with water.
- Place a flat piece of stiff cardboard over the mouth of the glass.
- Hold the cardboard firmly and turn the glass upside down (do this over a sink just in case!).
- Carefully remove your hand from the cardboard.
The cardboard will stay in place, holding the water in. This is because the air pressure pushing up from below is stronger than the weight of the water pushing down.
7. What are some other daily life examples of air pressure?
Air pressure is working in many situations you see every day. For example:
- Pumping air into a bicycle or car tyre.
- Using a suction cup to hang something on a window.
- Drawing liquid into a dropper or a syringe.
- The working of a vacuum cleaner to suck up dust.









