




How Are Chemical Mixtures Classified?
Today we will learn about Chemical mixtures. The most basic example of a mixture is the air we breathe. Do you realize that? We will study a little more into the impure materials, or mixes, as they're sometimes referred to. A chemical mixture is a mixture of two more than two substances or compounds. These substances are not bonded to chemicals. The mixtures of substances are in a fixed ratio. In this article, we will study the mixture and types of the mixture (Homogeneous Mixture and Heterogeneous Mixture) with examples. We will also discuss the Solution and the difference between mixture and solution.
Chemical Mixture
What is a Mixture in Chemistry?
In chemistry, a mixture is made up of two or more two chemical substances which are not bonded or linked to chemicals. A mixture is a physical combination in the form of Solution, Suspension, and Collides. In a mixture, the compounds can be easily separated and are variable. We can separate mixtures by using mechanical methods like purification, distillation, electrolysis, chromatography, heat, filtration, and gravitational sorting. There is no change or little change of energy. In other words, a mixture is something you obtain when you combine two things, allowing you to separate them again without causing a chemical reaction.
Types of Mixture
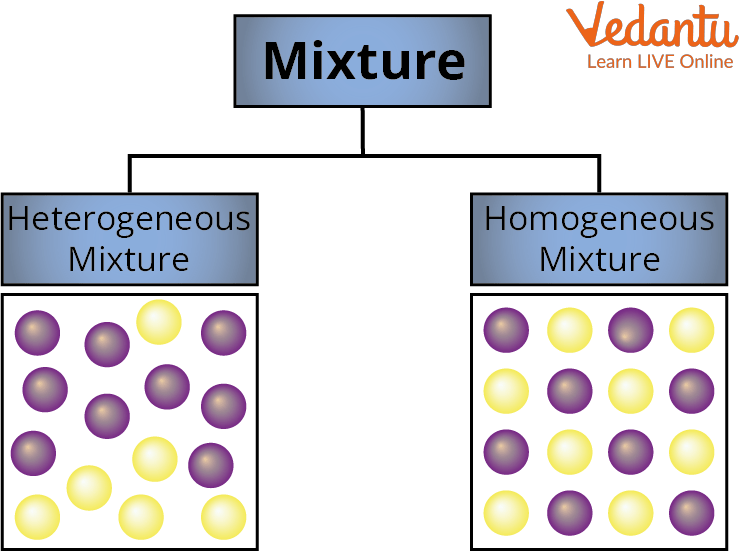
Types of Mixture
Mixtures are classified into Homogenous Mixture and Heterogeneous Mixture
1. Homogeneous Mixture –
It is a mixture in which the substances are mixed together so well that the different parts do not settle on their own. Solid, Liquid, and Gas are homogenous mixtures. The mixing of salt and water is the best example here. Salt and water cannot be separated at this point because the path of light is invisible when a ray of light strikes a solution of salt and water. In a homogenous mixture, the size of particles is less than 1 nanometre. A mixture is said to be homogenous if its composition is constant throughout. Because the dissolved salt is evenly distributed throughout the whole salt water, the saltwater described above is homogenous.
Example: Mixture of Salt and Water.
2. Heterogeneous Mixture -
It is a mixture in which the substances are mixed together but not dissolved together. All of its particles are visible with a microscope. The parts are simple to recognize, and more than one phase is visible with the unaided eye.
Example: Mixture of oil and Water.
Examples of Mixture
1. Mixture of Wood and Water
2. Mixture of flour and butter
3. Mixture of Sugar and tea
4. Mixture of petroleum and water
5. Mixture of Copper and Silver
6. Mixture of Zinc and Copper
7. Mixture of sand and water
8. Mixture of Nickel and Iron
Difference Between Mixture and Solution
Summary
Mixtures are the one product of combining different substances, elements, and compounds. Every substance in a combination maintains its unique chemical identity and this is understandable that mixtures are the result of mechanically blending the components or complex without any chemical bonding occurring, it means that the mixture has no chemical bonding, and all the elements engaged in the mixing process retain their original chemical characteristics.
FAQs on Chemical Mixtures: Meaning, Types & Examples
1. What is a chemical mixture in science?
A chemical mixture is a substance made by physically combining two or more different materials without a chemical reaction occurring between them. The individual substances in a mixture retain their own chemical properties and can be separated by physical means. For example, a mixture of sand and sugar can be separated as the components have not chemically bonded.
2. How is a chemical mixture different from a chemical compound?
A chemical mixture differs from a compound in several key ways. The primary distinction is that a mixture involves a physical blending of substances, whereas a compound involves a chemical bond.
- Formation: Mixtures are formed by physical mixing, with no energy change. Compounds are formed by a chemical reaction, often involving heat or light.
- Composition: Mixtures have a variable ratio of components (e.g., you can add more or less sugar to water). Compounds have a fixed ratio of elements by mass (e.g., water is always H₂O).
- Properties: Components in a mixture retain their original properties. A compound has new properties, different from its constituent elements.
- Separation: Components of a mixture can be separated by physical methods like filtration or evaporation. Elements in a compound can only be separated by chemical reactions.
3. What are the main types of mixtures, with examples?
Mixtures are primarily classified into two main types based on the uniformity of their composition:
- Homogeneous Mixtures: These have a uniform composition throughout, meaning the components are evenly distributed and cannot be seen separately. Examples include saltwater (salt dissolved in water), air (a mix of gases), and alloys like brass.
- Heterogeneous Mixtures: These have a non-uniform composition, and their components can be easily distinguished. Examples include sand in water, oil and vinegar salad dressing, and concrete (a mix of cement, sand, and gravel).
4. Why are mixtures important in our daily lives?
Mixtures are fundamental to our existence and technology. The air we breathe is a homogeneous mixture of nitrogen, oxygen, and other gases. The food we eat, like salads or soups, are heterogeneous mixtures. In industry, mixtures are crucial for creating materials like concrete, alloys (steel), and plastics. Even medicines are often formulated as mixtures to deliver active ingredients effectively.
5. Is blood considered a homogeneous or heterogeneous mixture?
While blood may appear uniform to the naked eye, it is technically a heterogeneous mixture. It is a type of colloid. This is because it contains various components like plasma, red blood cells, white blood cells, and platelets that are not chemically bonded and are not uniformly distributed. These components can be separated by physical means, such as using a centrifuge.
6. What type of mixture is steel, and how does its composition give it special properties?
Steel is a type of homogeneous mixture known as an alloy. It is primarily a mixture of iron and a small amount of carbon. Unlike pure iron, which is relatively soft and rusts easily, the addition of carbon creates a much stronger and more durable material. By varying the amount of carbon and adding other elements like chromium or nickel, different types of steel with specific properties (like stainless steel's rust resistance) can be created.
7. What is the difference between a solution, a suspension, and a colloid?
Solutions, suspensions, and colloids are all types of mixtures, but they differ based on the size of the particles dispersed within them and their stability.
- A Solution is a homogeneous mixture with very small solute particles (less than 1 nm) that do not settle out or scatter light. Example: Saltwater.
- A Suspension is a heterogeneous mixture with large particles (over 1000 nm) that are visible and will settle out over time if left undisturbed. Example: Muddy water.
- A Colloid is a type of heterogeneous mixture with intermediate-sized particles (1-1000 nm) that remain dispersed and do not settle. Colloids often scatter light, a phenomenon known as the Tyndall effect. Example: Milk or fog.
8. Can the components of a mixture be easily separated?
Yes, a defining characteristic of a mixture is that its components can be separated using physical methods that rely on differences in their physical properties. Because no chemical bonds are formed, these methods do not require a chemical reaction. Common separation techniques include:
- Filtration: To separate an insoluble solid from a liquid (e.g., sand from water).
- Evaporation: To separate a soluble solid from a liquid (e.g., salt from water).
- Magnetism: To separate a magnetic substance from a non-magnetic one (e.g., iron filings from sand).









