




Real-Life Examples of the Law of Conservation of Matter
The law of conservation of matter says that the amount of matter remains the same, in any event, when matter changes form. Sometimes it might appear to be that matter vanishes during a science experiment; however, this law lets us know that matter can't mystically appear or vanish; it just changes from one form to another. One more method for making sense of the law of conservation of matter is to express that things can't be magically destroyed. In short, “The mass in an isolated system can neither be created nor be destroyed, but it can be transferred from one form to another”.

Fire and Ash
What is the Law of Conservation of Matter?
The law of conservation of mass or principle of mass conservation expresses that for any system close to all transfers of matter and energy, the mass of the system should stay consistent over time, as system mass can't change the amount if it is not added or removed. Subsequently, the amount of mass is "conserved" over time. This law states that mass can neither be created nor be destroyed, although it might be rearranged in space, or the substances related to it could be changed in form, for example when light or physical work is changed into particles that contribute a similar mass to the system as the light or work had contributed.
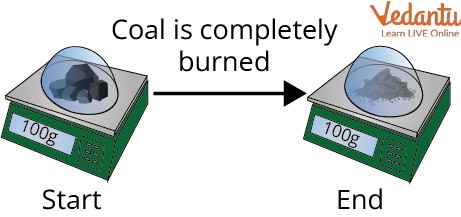
The Weight of Coal Remains the Same Even After Burning
Fire is which State of Matter?
Burning might destroy matter, but the same amount, or mass, of matter, actually exists after a campfire as before. It's like when wood burns, it combines with oxygen and changes not only to ashes but also to carbon dioxide and water vapour. The gases float off high in the air, leaving only the ashes. While there is some ionisation in a typical fire, the majority of the substance in the flame is a gas. As a result, the safest response to the question "Fire is which State of Matter?" is that it is a gas. Or, to put it another way, it's primarily gas with a minor quantity of plasma.
What are the Examples of the Law of Conservation of Matter?
The law of conservation of matter examples include:
Combustion Process: The burning of wood is a conservation of mass as the burning of wood includes oxygen, carbon dioxide, water vapours, and ashes.
Chemical Reactions: To get one molecule of H2O (water) with the weight of 10, hydrogen with molecular weight 2 is added with oxygen whose molecular weight is 8, in this way conserving the mass.
What is Conversion of Matter?
Conversion of matter refers to the change from one state of matter to another. It is a cycle by which matter changes starting with one state then onto the next and back to its original state, with no adjustment of its chemical composition. Solid can be converted into fluids by heating. Essentially, fluids can be converted into gases by heating and gases can be converted into fluid and fluids can be converted into solids by cooling. The conversion of states is a physical change because these changes happen without a change of composition and no change in the chemical nature of the substance.
Solid to Liquid: Melting
Liquid to Gas: Evaporation
Gas to Liquid: Condensation
Liquid to Solid: Freezing
Solid to Gas: Sublimation
Gas to Solid: Deposition
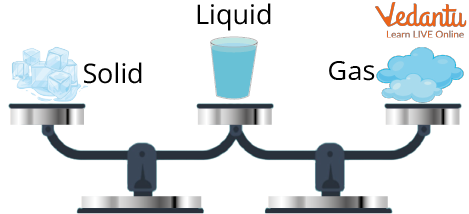
Weight Remains the Same in any Form
Summary
In this article, we learnt about the law of conservation of matter by observing our day-to-day life examples like burning and different changes in the matter do not destroy matter. The mass of matter is consistently the same before and when the changes happen. The law of conservation of mass expresses that matter cannot be created or destroyed.
FAQs on Law of Conservation of Matter Explained for Students
1. What is the Law of Conservation of Matter in simple terms for kids?
The Law of Conservation of Matter states that matter cannot be created or destroyed, it only changes its form. Think of it like playing with LEGO bricks. If you have a pile of 100 bricks, you can build a car, then take it apart and build a house. You still have the same 100 bricks you started with; they've just been rearranged.
2. Can you give a simple example of the Law of Conservation of Matter?
A perfect example is melting an ice cube. If you weigh an ice cube and then let it melt into water, the water will have the exact same weight as the ice cube did. No matter was lost; it just changed from a solid to a liquid. The amount of 'stuff' (mass) stays the same.
3. Is the Law of Conservation of Matter the same as the Law of Conservation of Mass?
Yes, in the context of school science, these two laws are used to describe the same principle. Matter is anything that has mass and takes up space. The law essentially says that the total amount of mass in a closed system will remain constant, no matter what physical or chemical changes occur.
4. When a log burns and turns into a small pile of ash, it seems like matter has been destroyed. How does the law explain this?
This is a great observation and a common point of confusion. The matter isn't actually destroyed. When the log burns, it changes into several things. If you could capture and weigh everything produced, you would find that:
- The weight of the original log
- The weight of the ash + the weight of the smoke + the weight of the invisible gases (like carbon dioxide and water vapour) released into the air.
5. Why is the Law of Conservation of Matter important in our daily lives?
This law is fundamental to understanding the world around us. It explains why following a recipe works in baking—the ingredients combine to form a cake of the same total mass. It's also the principle behind recycling, where we take old materials like plastic bottles and turn them into new items without losing the material itself. It even applies to how our bodies use food, converting it into energy and waste products without destroying any matter.
6. How does this law apply to both physical and chemical changes?
The law holds true for both types of changes. In a physical change, like water freezing into ice, the substance (H₂O) remains the same, and its mass does not change. In a chemical change, like baking a cake, new substances are formed, but the total mass of the reactants (flour, eggs, sugar) equals the total mass of the products (the cake and any evaporated water). The atoms are simply rearranged to make new molecules, but no atoms are ever lost or gained.









