




Key Properties and Uses of Lead for Students
In school, we write with the help of a pencil. Every individual has used a pencil in their childhood. But have you ever thought about how we can use and write with the help of a pencil? What makes a pencil have such properties?
The answer to this is lead; with the help of a lead, we are able to write. Also, lead is known as Plumbum (chemical name of lead). Let's look at the picture below to recall the memories of using a pencil.

Pencil Having Lead
Description of Lead
Lead is a chemical element with symbolic representation as Pb, and its atomic number is 82. Lead is a heavy metal that is denser than most other materials. Lead is soft and is also malleable in nature. Lead has a low melting point.
Lead is silvery white or grayish metal in color. It is placed in group 14 on the periodic table. It is malleable, ductile, and dense in nature. Also, it is a poor conductor of electricity.
It contains only one type of atom in it. It is a chemical substance that is very resistant to corrosion but if exposed to air it gets tarnished easily. Lead is also used in homes and around us, which includes paint, ceramics, pipes, and also in plumbing materials, and cosmetics.
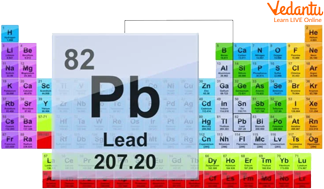
Position of Lead in the Periodic Table
Properties of Lead
Lead has an atomic mass of 207.2. Its melting point is 327 C and its boiling point is 1755 C. It has 13 isotopes.
The metal in its powdered form produces a bluish-white flame when burnt in the air.
Lead has an ionic radius of 0.840 A.
So, Properties of Lead Can be Summed Up As:
The properties of lead that make it useful in a wide variety of applications are density, malleability, lubricity, flexibility.
Lead has good resistance to corrosion.
Lead is an easier alloy than many other metals and casts with little difficulty.
Lead has quite low elasticity, strength, and hardness.
Uses of Lead
Lead is used in car batteries.

Lead in Car Batteries
Lead is used in roofing, and as a cover for electric wires.

Lead Used in Wires as Covering
It is used in shipbuilding as it is resistant to corrosion.
Lead is used in cable sheaths.
It is also used as a coloring agent.
Lead is also used in making crystal glass and is commonly known as crystal. Lead glass contains typically 18-40% lead (II) oxide, while modern lead crystals known as flint glass contain a minimum of 24% of PbO.

Lead Crystal Glass
Summary
Lead has been known since ancient times. Lead is made from a form of carbon called graphite which is mixed with clay and formed into lead used in pencil. Moreover, this element is found in nature in pure form but in ores. The natural formation of lead is by radioactive decay of uranium and thorium. Also, it reacts more rapidly with hot acids and is slow reactive in water and cold acids.
Its ores are widely distributed and it has a low melting point. Thus, it melts easily and has a lot of uses. It is found in all parts of our environment, and much of it comes from human activities. Lead has been extracted by roasting the ore and then smelting it. Lead in industries is called a useful metal, it has a lot of commercial purposes and usage.
FAQs on Lead in the Periodic Table
1. What are the properties of lead?
Lead is very malleable, ductile, and dense in nature. Apart from this, lead is a poor conductor of electricity. Moreover, it has a low melting point and it is one of the most stable metals that do not rust. Lead does not react with water. Its element name is Pb and has the atomic number 82. Its atomic weight is 207.2 C. It is a basic metal. These are some of the vital properties of lead.
2. What happens when lead metal is touched?
According to certain research, lead can be absorbed into the skin. You could be exposed if you handle lead and then touch your lips, nose, or eyes. Additionally, lead dust can contaminate your hair and clothing. If this occurs, it's probable that you'll bring some lead dust home with you, exposing your family as well.
3. Does lead rust?
Among the most stable metals which do not rust is lead. But similar to zinc, lead can also be oxidized. Lead is used in a wide variety of applications.
4. Write some interesting facts about Lead.
Interesting facts about lead are:
Lead is one of the metals that is known to ancient man.
Lead is highly toxic in nature.
Lead is the only metal that has zero Thomson effect.
Lead is present in abundance in the earth’s crust and is 14 parts per million by weight.
The name lead is derived from a Latin word called ‘Plumbum’.
Ancient Egyptians were the first ones to extract lead which they used to make sculptures.









