




Real-Life Applications and Benefits of Sulphur in EVS
A naturally occurring element called sulphur encourages the more effective use of Earth's resources. It is a vital essential nutrient in agriculture, a crucial component of sustainable urban development, as well as a crucial component in initiatives to halt the loss of arable farmland due to soil degradation.
In this article, we will read about sulphur. Towards the end, we will be aware of 10 uses of sulphur and almost everything important information about sulphur. We will also discover the significance that sulphur plays in our daily lives.
What is Sulphur?
Sulphur is a plentiful, multivalent non-metal that has no flavour or odour. Sulphur is a yellow solid substance in its natural state. It can be found in nature either as a pure metal or as sulphide and sulphate minerals. Although sulphur is notorious for its stench, which is commonly compared to rotten eggs, hydrogen sulphide has that scent (H2S).
Pure sulphur is a tasteless, odourless, rigid solid with a pale yellow colour that is insoluble in water and a poor conductor of electricity. It creates sulphides when it combines with all metals, excluding gold and platinum, and it also does so when it interacts with a number of nonmetallic elements.
Sulphur crystallography is intricate. Sulphur allotropes can produce a variety of unique crystal forms depending on the circumstances. Sulphur may have made it feasible for life to exist on Earth. The variety of amino acids that make up the basis of life might have been produced by straightforward chemical reactions earlier, in the seas.
Sulphur naturally occurs close to volcanoes. Massive amounts of native sulphur are found naturally in Texas and Louisiana in the United States. Numerous sulphide minerals are recognised, including sphalerite, zinc sulphide, stibnite, antimony sulphide, galena, lead sulphide, pyrite, and marcasite, which are both iron sulphides. Chalcopyrite, bornite, pentlandite, millerite, and molybdenite are additional, more significant sulphide ores.

Sulphur Physical Form
Uses of Sulphur
In addition to being a fungicide and an ingredient in black gunpowder, sulphur is used for vulcanising black rubber. However, the majority of sulphur is utilised in the creation of sulfuric acid, which is arguably the most significant chemical produced by western civilisations. The production of phosphoric acid to create phosphates for fertilisers is the most significant of sulfuric acid's various applications.
A class of organosulfur compounds is known as mercaptans. Some of these are supplied to natural gas supply so that leaks can be quickly discovered due to their particular odour. Others are employed in the creation of insecticides and herbicides as well as in the polishing of silver.
Sulfites are used in many foods as preservatives and to bleach paper. Sulphate derivatives are present in many surfactants and detergents. For usage in cement and plaster, 100 million tonnes of calcium sulphate (gypsum) are mined annually.
Uses of Sulphur in Plants
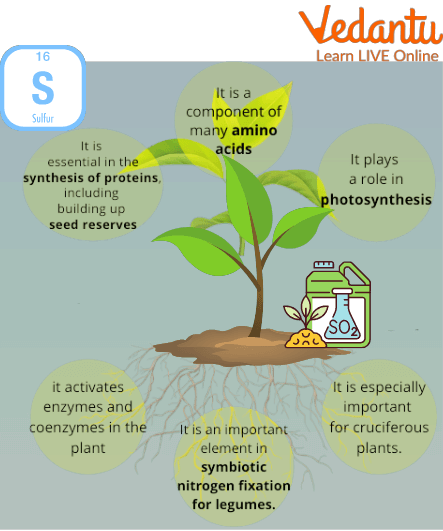
Role of Sulphur in Plants
Numerous plant growth processes, such as nitrogen fixation, enzyme activity, protein and oil synthesis, depend on sulphur. Sulphur-deficient plants typically feature short and/or lanky stems, and young (top) leaves that have yellowed. When there is a nitrogen shortage, the older leaves are the first to yellow. In addition, immature leaves may purpling and cup upward, flowering may be delayed and extended, the flowers may be pale in colour, and the pods may be fewer and smaller in sulphur-deficiency canola.
Uses of Sulphur for Skin
All living tissues include the chemical element sulphur. It ranks third among the most prevalent minerals in the human body, behind calcium and phosphorus. Broccoli, onions, and garlic also contain sulphur.
Shortness of breath, allergies, pharyngitis (swelling at the back of the throat), heart problems, blocked arteries, menopausal, and respiratory tract infections like the cold virus are all treated with sulphur taken orally.
Sulphur is administered topically to the skin to treat conditions like rosacea, dandruff, scabies, lice, cold sores, warts, and infections from poison oak, ivy, and sumac in addition to acne, hay fever, skin redness, acne, and dandruff.
Summary
Sulphur is a non-metallic chemical element with the atomic number 16 and the chemical symbol S It is extremely reactive yet can be found naturally in deposits, coupled in other ores (such as pyrite, galena, and cinnabar), in coal, petroleum, and natural gas, as well as in sulphur spring water.
Sulphur is one of the four most significant basic chemical commodities and the third most common mineral component. One of the largest industrial uses of sulphur is that it is used in making phosphate fertilisers. We will wrap up this article here only.
FAQs on Uses of Sulphur Explained for Students
1. What is the single most important industrial use of sulphur?
The primary and most significant industrial use of sulphur is in the production of sulphuric acid (H₂SO₄). This compound is so crucial that its production volume is often seen as an indicator of a nation's industrial strength. A vast majority of the produced sulphuric acid is then used to manufacture phosphate fertilisers, which are essential for modern agriculture as per the 2025-26 CBSE syllabus.
2. What are ten important uses of sulphur across different sectors?
Sulphur is a versatile element with numerous applications. Ten important uses include:
- Manufacturing sulphuric acid, the world's most-produced chemical.
- Production of fertilisers, especially superphosphates, to support crop growth.
- Vulcanisation of rubber to enhance its strength and durability.
- Making fungicides and pesticides for agriculture.
- Manufacturing of matches and fireworks (in black gunpowder).
- Production of carbon disulphide, a solvent for waxes and resins.
- Use in detergents and surfactants as sulphate derivatives.
- Treatment of skin conditions like acne and dandruff in medicinal preparations.
- Bleaching paper and preserving food using sulfites.
- Creation of certain dyes and pigments.
3. What is the role of sulphur in plant nutrition and agriculture?
Sulphur is an essential macronutrient for plants, often called the 'fourth major nutrient' after nitrogen, phosphorus, and potassium. It is a critical component of essential amino acids (cysteine and methionine), which are the building blocks of proteins. Sulphur also plays a vital role in chlorophyll formation, nitrogen fixation in legumes, and the activation of various enzymes necessary for plant metabolism and oil synthesis.
4. How is sulphur used in skincare and medicine?
In medicine and dermatology, sulphur acts as a keratolytic agent, meaning it helps to shed the outer layer of the skin. This property makes it effective in topical treatments for various skin conditions. It is commonly used in ointments, lotions, and soaps to treat acne by drying out the skin and helping to unclog pores. It is also used in preparations for dandruff, scabies, and rosacea due to its antibacterial and anti-inflammatory properties.
5. Does pure sulphur have the characteristic 'rotten egg' smell?
No, pure, solid sulphur is actually odourless and tasteless. The infamous 'rotten egg' smell is not caused by elemental sulphur itself but by one of its compounds: hydrogen sulphide (H₂S). This gas is often produced in natural processes where sulphur compounds are present, such as in volcanoes or the breakdown of organic matter, leading to the common but incorrect association of the smell with pure sulphur.
6. How does sulphur's role in vulcanisation improve the quality of rubber?
Vulcanisation is a chemical process that transforms soft, sticky natural rubber into a durable, elastic material. During this process, sulphur atoms form cross-links between the long polymer chains of the rubber molecules. These cross-links prevent the polymer chains from sliding past each other when stretched, allowing the rubber to return to its original shape. This makes the rubber much stronger, more elastic, and more resistant to temperature changes.
7. Why is sulphur considered a vital element for both life and industry?
Sulphur's importance stems from its unique chemical properties and reactivity. For life, it is a fundamental component of essential amino acids (methionine and cysteine), which are necessary to build proteins in all living organisms. It is also part of crucial vitamins like thiamine and biotin. For industry, its role in creating sulphuric acid makes it indispensable for manufacturing fertilisers, chemicals, and pigments. This dual role—as a building block for life and a cornerstone of the chemical industry—makes it uniquely vital.
8. What are the key uses of sulphur dioxide, and what are its environmental impacts?
Sulphur dioxide (SO₂) is primarily used as a precursor to sulphuric acid via the Contact process. It also has significant uses as a food preservative (preventing browning in dried fruits), a disinfectant, and a bleaching agent for paper pulp and textiles. However, sulphur dioxide is also a major air pollutant. When released from burning fossil fuels or industrial processes, it can react with water and other substances in the atmosphere to form acid rain, which harms forests, aquatic life, and buildings.
9. What are the different allotropic forms of sulphur mentioned in the NCERT syllabus?
Sulphur exhibits several allotropic forms due to the different ways its atoms can bond. The two most important crystalline allotropes as per the NCERT syllabus are:
- Rhombic sulphur (α-sulphur): This is the stable form at room temperature. Its crystals are yellow and have an octahedral shape. The S₈ molecules are packed in a puckered, crown-shaped ring.
- Monoclinic sulphur (β-sulphur): This form is stable above 369 K. It consists of the same S₈ puckered rings but has a different crystal packing, resulting in long, needle-shaped crystals. It slowly reverts to the more stable rhombic form below this temperature.









