




Introduction
A typical solvent in various medical technologies and various business related labs is used and is called as xylene. The French chemist Auguste Cahors (1813-1891) discovered xylene. The primary consequence of breathing in xylene vapour is central nervous system (CNS) depression, which causes headache, nausea, dizziness, and vomiting. Meta-xylene, ortho-xylene, and para-xylene are the three different types of xylene. These three xylene isomers are together known as complete xylenes. Xylene evaporates very quickly, which is why most of the xylene which enters the soil or water again goes into the atmosphere. The most common sources of xylene emissions include industrial processes and vehicle exhaust when xylene is used as a solvent.
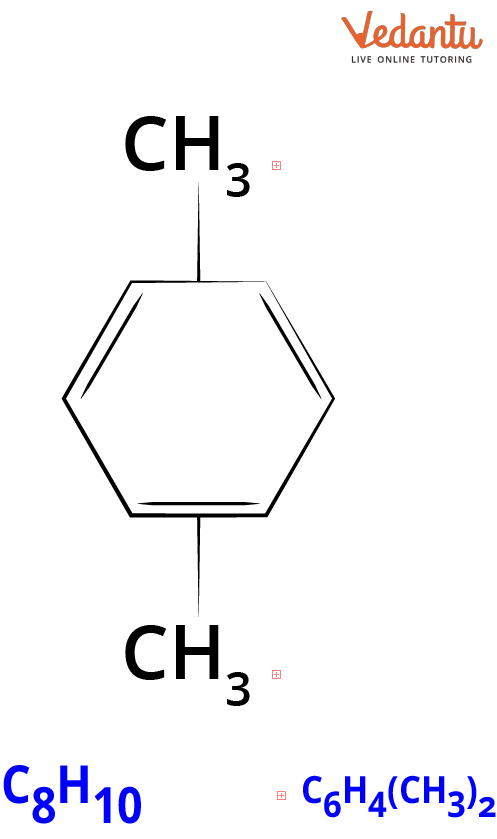
Structure of Xylene in 2-dimensional Form.
What is Xylene?
The colourless liquid known as xylene smells delicious. Xylene burns easily and is insoluble. Although xylene is a synthetic substance, xylene can sometimes occur naturally in coal tar, oil, or during forest fires. In Greek, its name is Xylong.
Xylene has the chemical formula C8H10, or more precisely (C6H4)(CH3)2. Dimethyl benzene, methyl toluene, xylol, and mixed xylenes are other terms for xylene. A six-carbon ring and two methyl groups make up the structure of xylene. Xylene is heavier than air.
Properties of Xylene
Commercially available xylene is a liquid that is poisonous, colourless, flammable, and non-viscous.
Although it is miscible with different organic liquids, it does not dissolve in water.
Industrial-scale xylene production involves the methylation of benzene and toluene.
Xylenes produce chemicals, agricultural sprays, adhesives, and coatings.
Xylene Stored in a Transparent Glass Jar.
Uses of Xylene
One of the essential compounds in the United States is xylene, which is frequently made from crude petroleum. Xylene has a wide range of applications, both on its own and in mixtures. It is widely used in various fields, including the dental and medical fields.
Xylene Uses in Paint- Specific oil-based paints, varnishes, adhesives, anti-rust, porch & deck paints, and synthetic enamels can all be thinned with xylene. Xylene is an excellent solvent for cleaning instruments and equipment immediately after use and is also effective at removing some adhesives.
Xylene Industrial Uses- In industries, xylene is used as a reagent which does the work of solvent in manufacturing various types of chemicals.
Xylene Uses in Food- Xylene gets present in food items due to environmental exposure because of transportation or handling. Some foods contain xylene, such as meat products, poultry, eggs, milk, bread, potatoes, etc.
Risks in Xylene
While xylene has many beneficial applications in various industries, those who work closely with this chemical molecule face specific hazards.
Children must be extremely cautious about xylene exposure since they are more vulnerable than adults.
Effects from inhalation or contact with the skin or eyes are the primary health hazards associated with xylene exposure.
Additionally, xylene irritates skin and eyes and can offer hazards to both.
Major organs may be impacted by smelling them.
Precautions to Take While Handling Xylene
Keep unneeded and exposed employees away from the spill location.
Use equipment that is grounded and explosion-proof.
Containment and cleanup techniques: Small leaks or spills should be contained and absorbed using an absorbent that won't react with the leaked product.
Conclusion
A colourless (C8H10), flammable liquid with a sweet smell is called xylene. Eyes, noses, throats, and skin might become irritated when exposed to xylene. Additionally, xylene can result in headaches, nausea, confusion, loss of coordination, and, in extreme cases, death. Workers who are exposed to xylene risk injury.
FAQs on Know the Chemical Xylene
1. Is xylene toxic and harmful?
Yes, xylene is highly hazardous to people. It is also known that this substance is combustible. It should be mentioned that exposure to xylene can cause depression in the central nervous system in humans and most other animals.
2. How is xylene produced?
Toluene and benzene are often methylated to produce xylene and its derivatives. The average composition of commercial or laboratory-grade xylene is 40–65% m-xylene, up to 20% o–xylene, p–xylene, and up to 20% ethylbenzene.
3. What happens if you touch xylene?
Inflammation and defatting of the skin can be brought on by xylene, especially after prolonged or recurrent contact with the liquid. Blisters and skin redness are possible side effects. Children are more susceptible to toxicants absorbed through the skin because their body weight to surface area ratio is substantially more considerable.









