




Introduction to the Mesomeric Effect
The mesomeric effect is the polarity created between atoms of a conjugated system via electron transfer or pi–bond electron transfer. In simple terms, the mesomeric effect happens when electrons in a conjugated orbital system move away from or towards a substituent group.
The effect, which is symbolised by the letter M, is used in a qualitative way to explain the electron withdrawing or releasing properties of substituents based on relevant resonance structures. Here, we will discuss the mesomeric effect, its type, and their order, as well as the resonance effect. We will also look into the differences between inductive and mesomeric effects.
Mesomeric Effect Order
There are two types of mesomeric effects namely-
(+M) i.e. positive mesomeric effect, which is when an electron-donating group is attached to the chain/ring.
(-M) i.e. negative mesomeric effect, which is when an electron-withdrawing group is attached to the chain/ring.
+M Effect Order :
$-\mathrm{O}^{-}>-\mathrm{NH}_{2}>-\mathrm{OR}>-\mathrm{NHCOR}>-\mathrm{OCOR}>-\mathrm{Ph}>-\mathrm{CH}_{3}>-\mathrm{F}>-\mathrm{Cl}>-\mathrm{Br}>-\mathrm{I}$
-M Effect Order :
$\begin{align} &-\mathrm{NO}_{2}>-\mathrm{CN}>-\mathrm{SO}_{3} \mathrm{H}>-\mathrm{CHO}>-\mathrm{COR}>-\mathrm{COOCOR}>-\mathrm{COO }>-\mathrm{COOH}>-\mathrm{CONH}_{2}> \\ &-\mathrm{COO}^{-} \end{align}$
Resonance Effect
When a single structure cannot represent all the properties of a molecule, then more than one different structure is used to explain all these properties, known as resonating structures or canonical form.
Resonating structures are hypothetical.
The real structure is somewhere between all these resonance structures and is called a resonance hybrid.
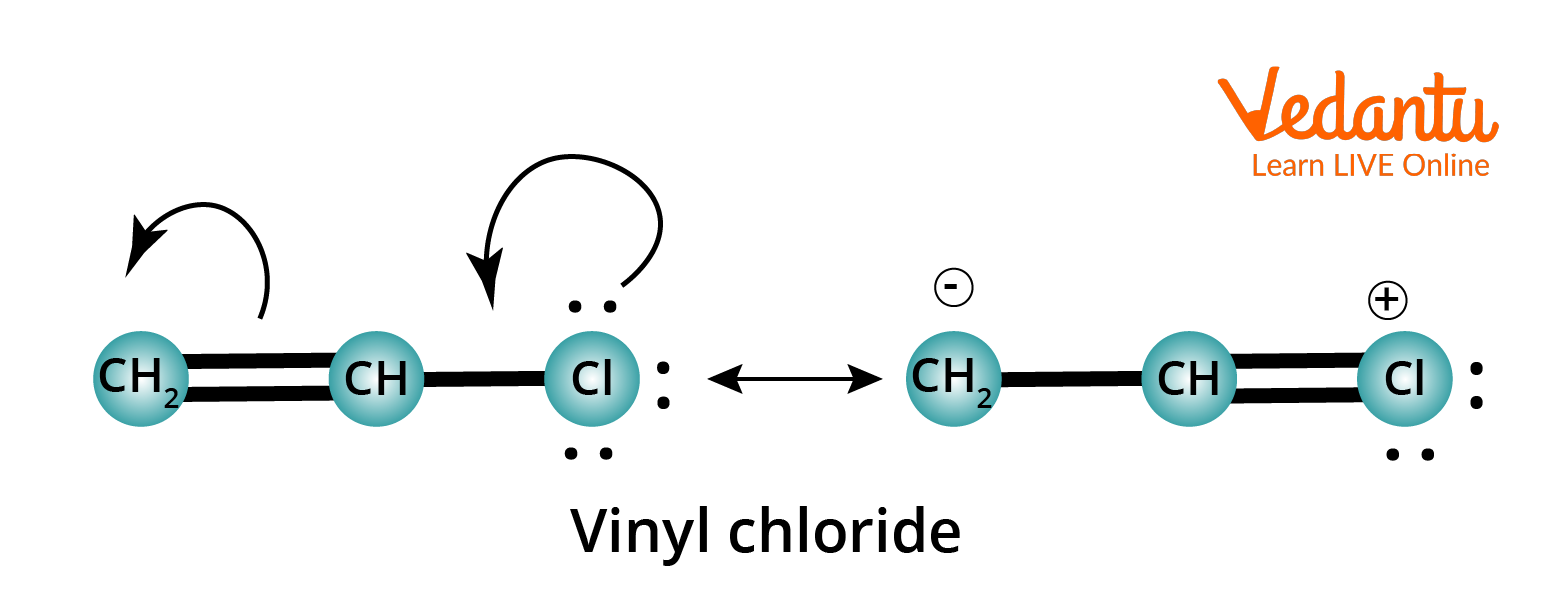
Resonance Structure of Vinyl Chloride
Positive Mesomeric Effect
The (+M) effect or positive mesomeric effect occurs when electrons or pi electrons are moved from a specific group to a conjugate system, hence boosting the electron density of the conjugated system.
The electrons are here because of the lone pair or (–ve) charge in the first atom's p orbital are given to the conjugated system at a distance.
Further linked atoms or groups will increase the +M effect if they are pushed by the +I or +M effect, but will lower the +M effect if they are pushed by the -I or -M effect.
The order of the +M impact between the following groups, for example, is
$-\mathrm{NH}-\mathrm{CH}_{3}>-\mathrm{NH}_{2}>-\mathrm{NH}-\mathrm{COCH}{ }_{3}>-\mathrm{N}\left(\mathrm{CH}_{3}\right)_{2}$
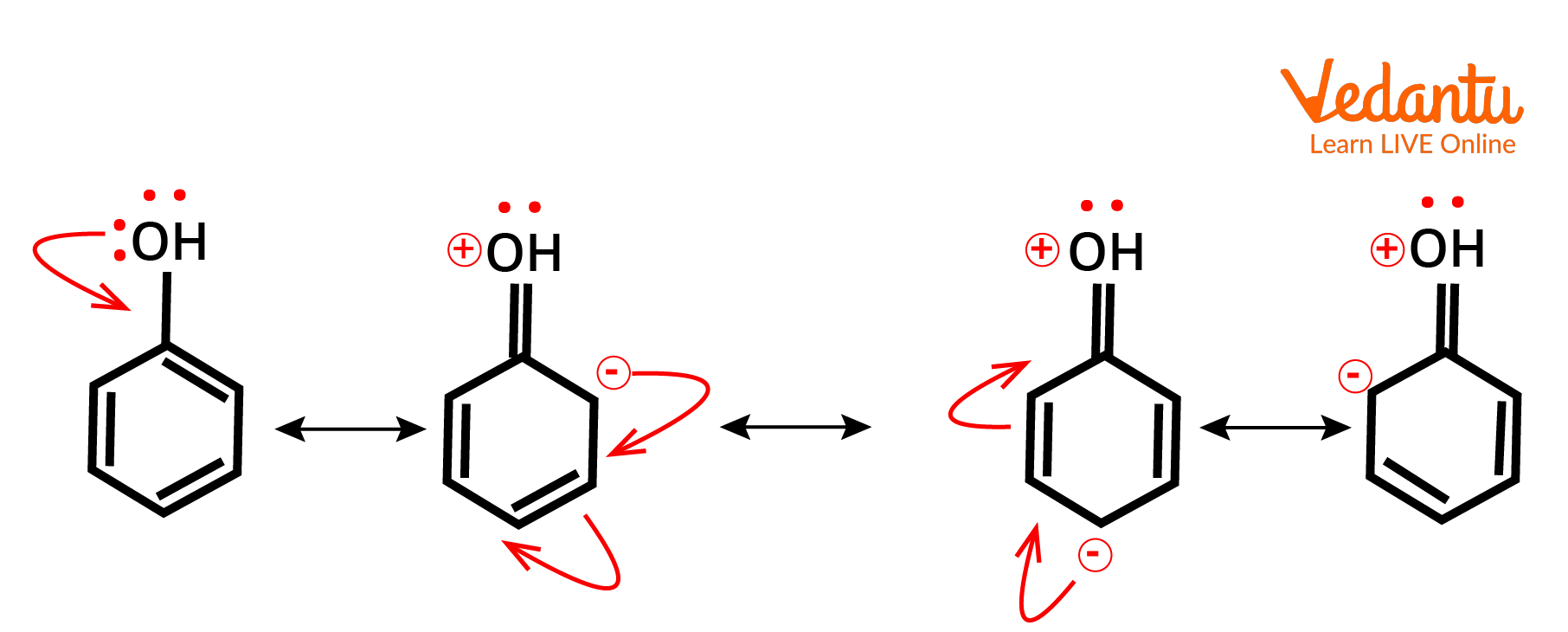
Example of Positive Mesomeric Effect
What is Resonance Mesomeric Effect?
Definition
Resonance Effect- The interaction between lone electron pairs and bond electron pairs causes resonance, which characterises the polarity of a molecule.
Mesomeric Effect- The influence of substituted or functional groups on chemical compounds is known as the mesomeric effect.
The primary distinction between resonance and mesomeric effect is that resonance is caused by the interaction of lone electron pairs and bond electron pairs, whereas mesomeric effect is caused by the presence of substituents or functional groups.
Difference Between Inductive effect and Mesomeric effect
Mesomeric Effect Examples
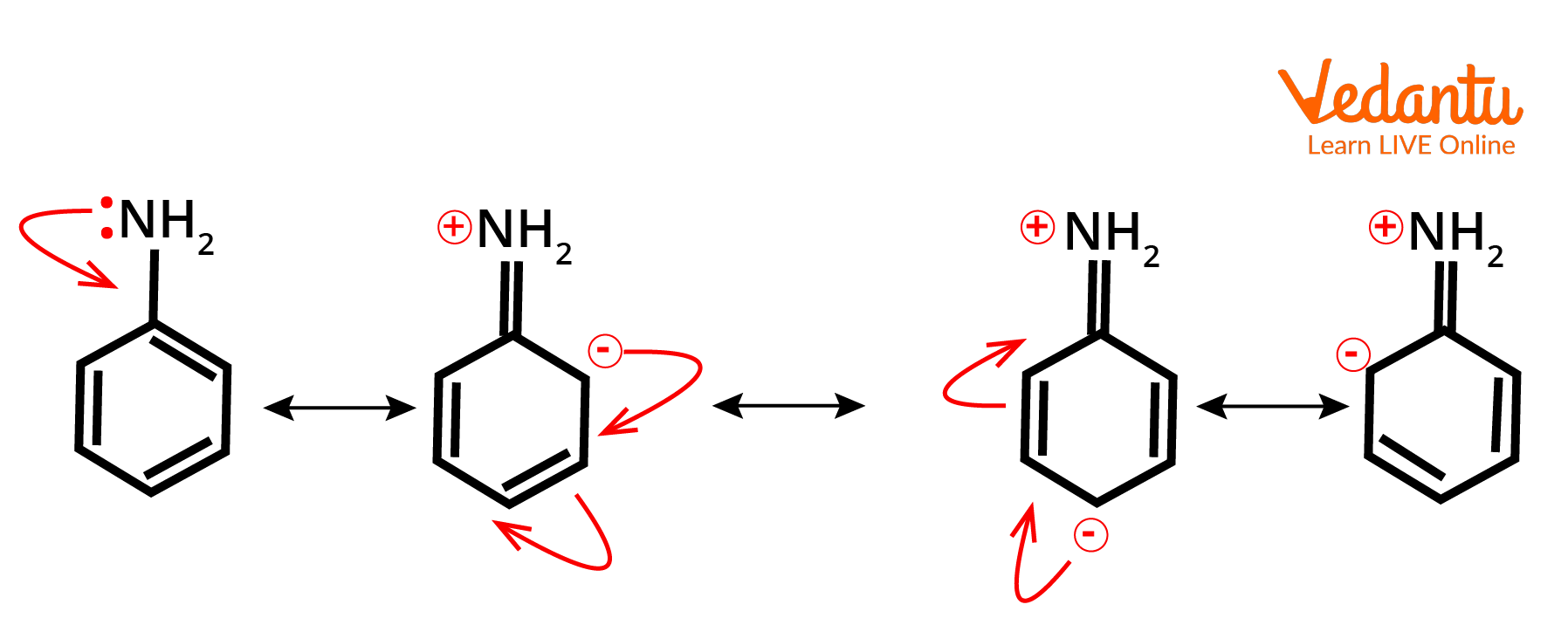
Example of +M effect
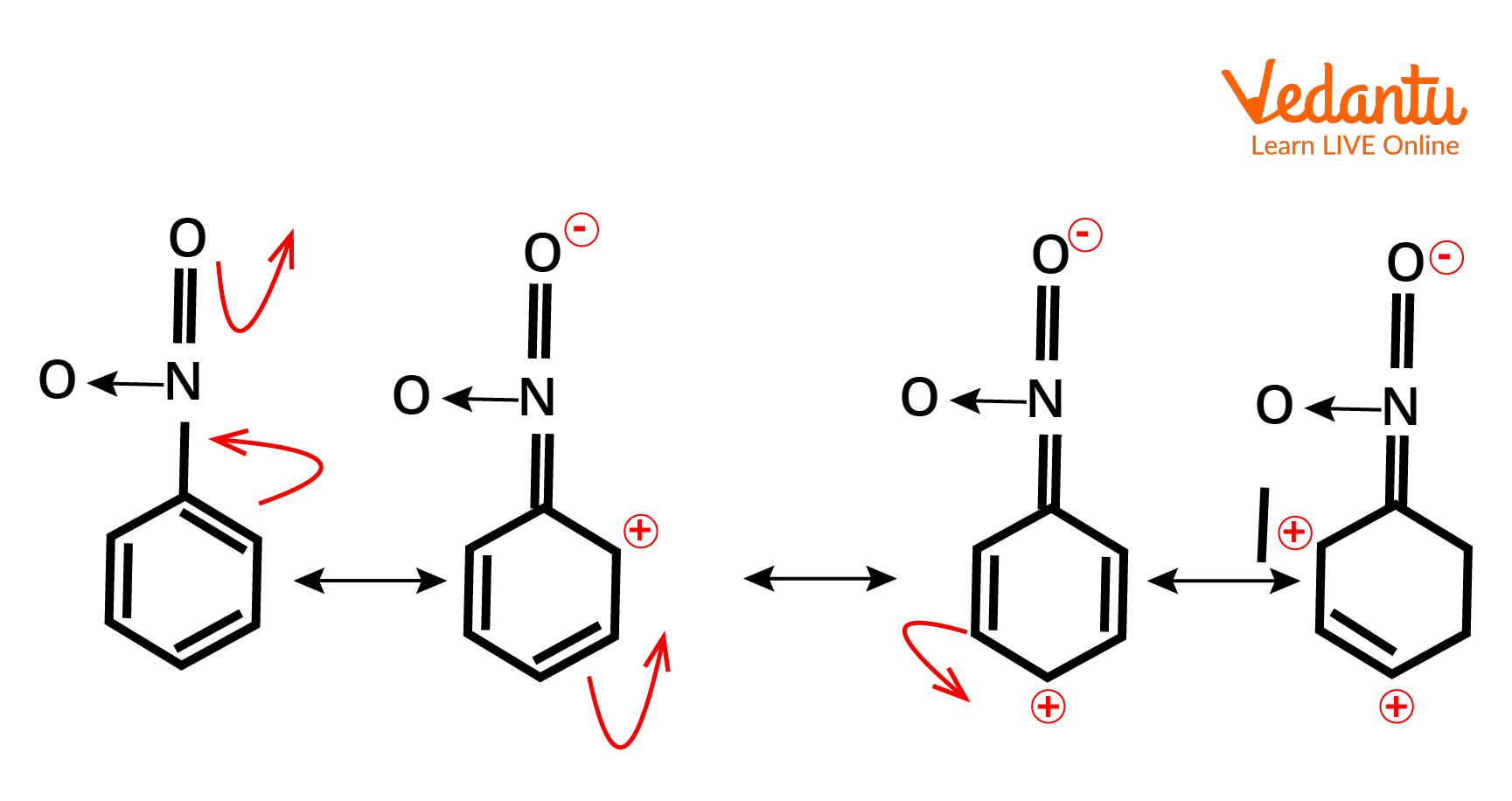
Example of -M effect
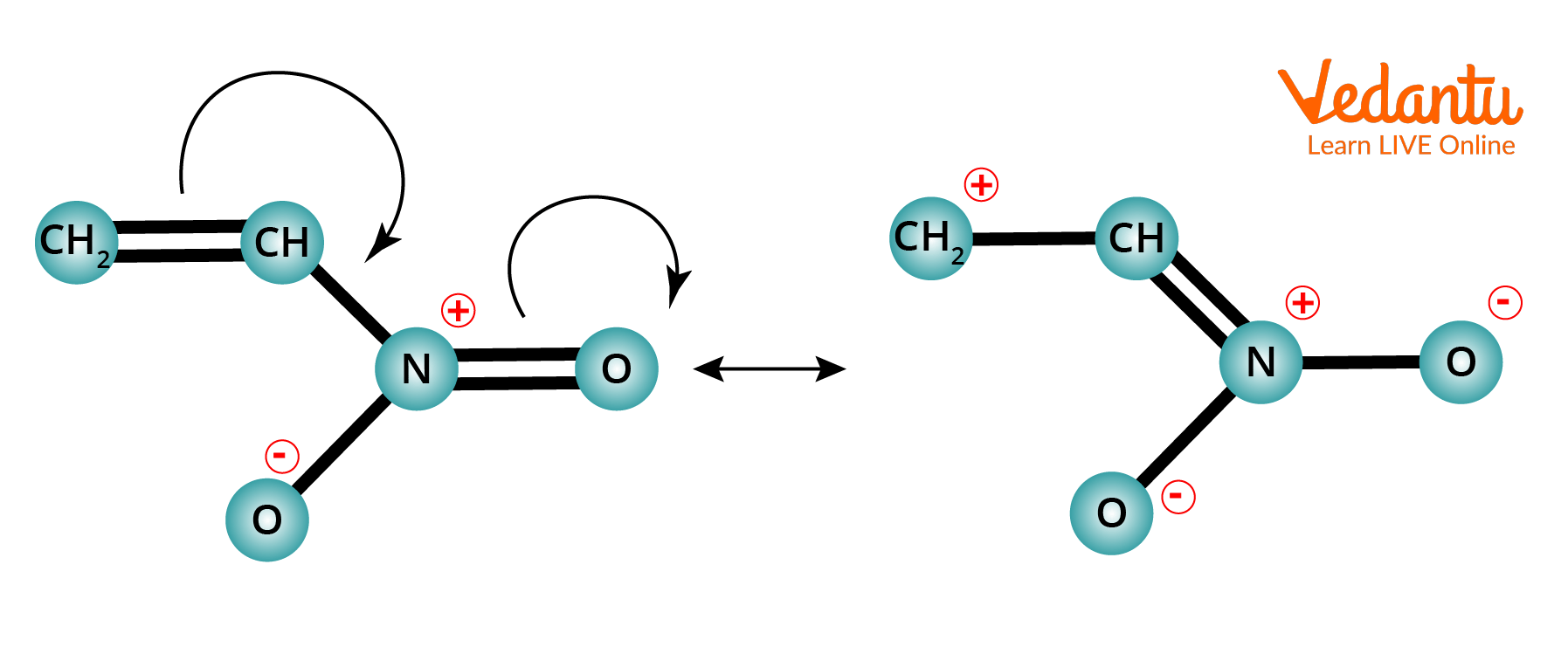
Example of -M Effect
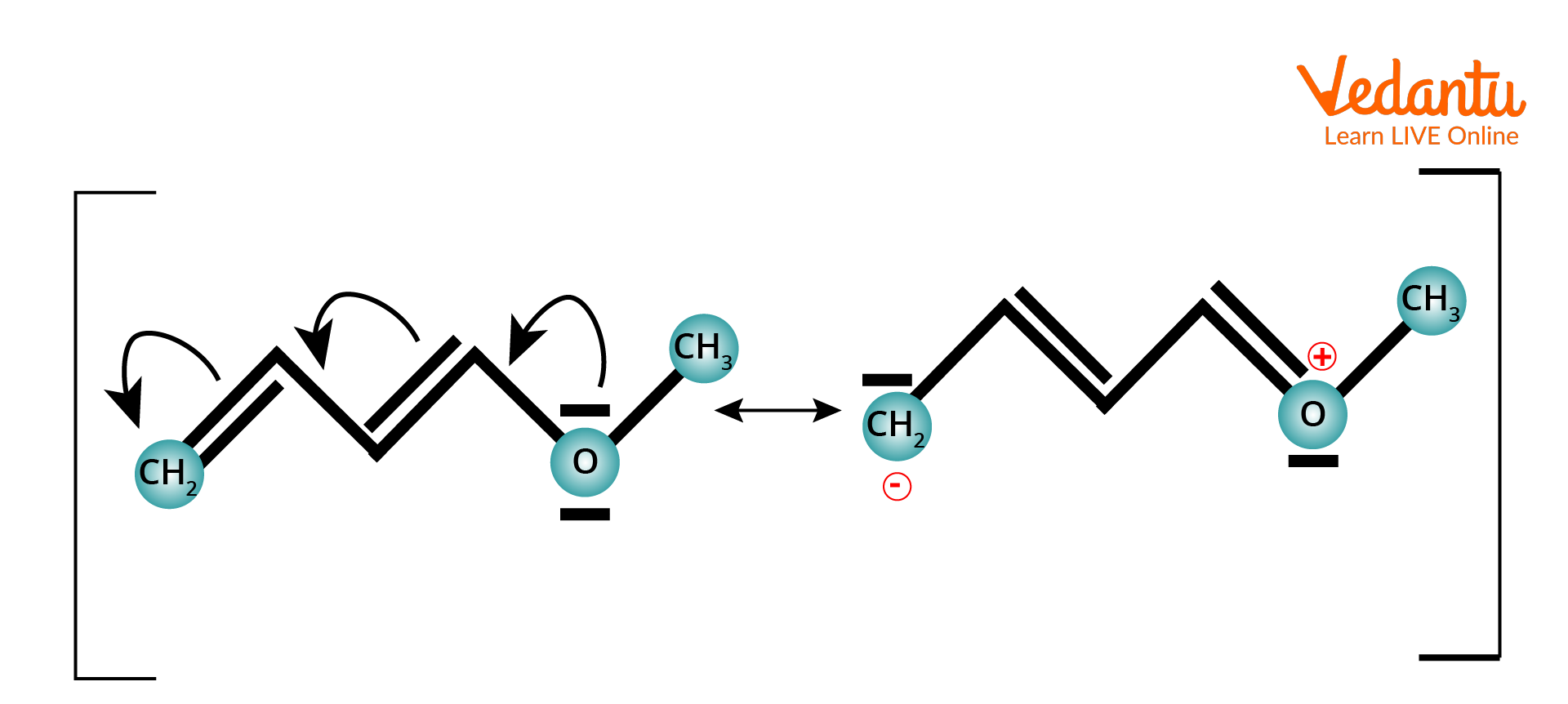
Example of +M Effect
Resonance Effect and Its Examples
Let’s explain the resonance effect with an example. A group of two or more Lewis structures that collectively represent the electronic bonding of a single polyatomic species, including fractional bonds and fractional charges, is known as a resonance structure. Resonance structures can be used to describe delocalized electrons that can't be described by a single Lewis formula with an integer number of covalent bonds.
Resonance structure of carbonate ion (CO3-2)
The carbonate ion's electronic structure, like that of ozone, is not represented by a single Lewis electron structure. Unlike O3, however, CO32- real structure is a composite of three resonance structures.
We put carbon in the middle since it is the least electronegative element.
There are 4 valence electrons in carbon, 6 valence electrons in each oxygen, and 2 extra for the 2 charges 4 + $3\times6$ + 2 = 24 valence electrons are obtained.
Between the oxygen atoms and the carbon atoms, six electrons are employed to form three bonding pairs.
By placing three lone pairs on each oxygen atom and marking the 2 charges, we split the remaining 18 electrons evenly among the three oxygen atoms.
The core atom has no electrons left.
Since the carbon atom only has 6 valence electrons at this point, we must use one lone pair from oxygen to build a carbon–oxygen double bond. However, in this scenario, three options are available.
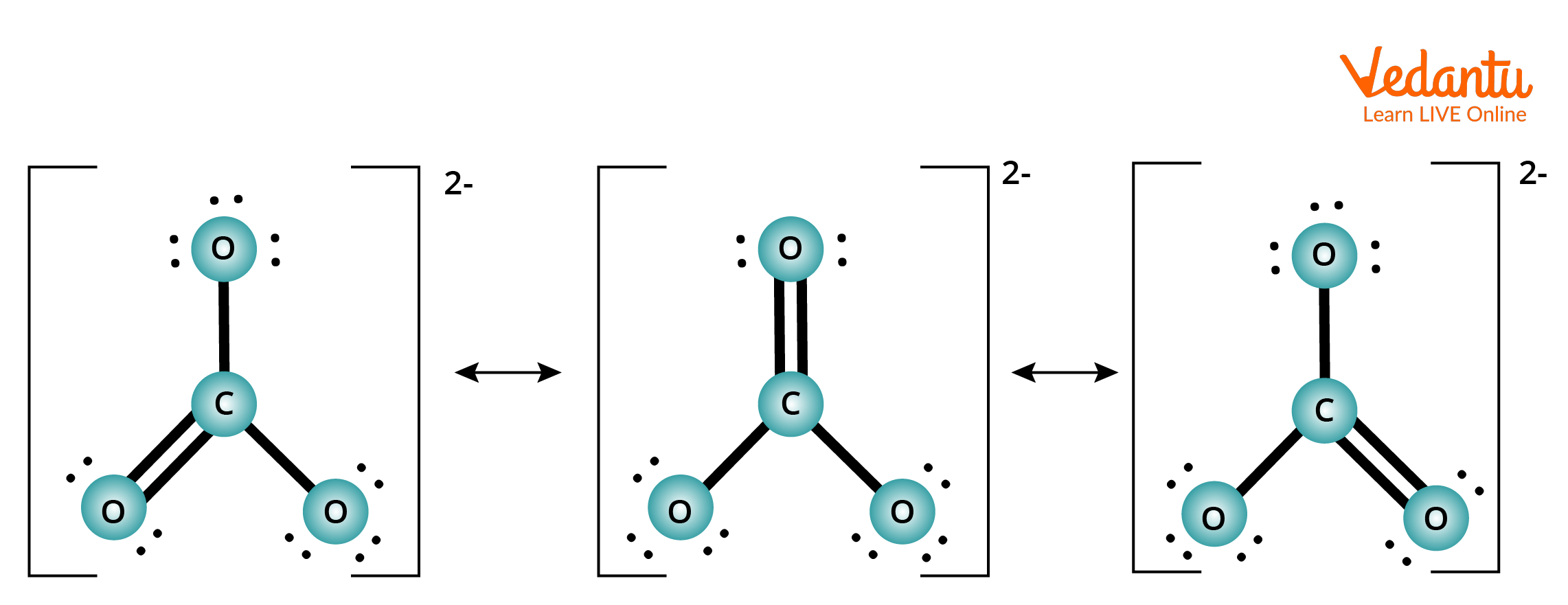
Example of Resonance Effect of Carbonate Ion.
Conclusion
One of the features of functional groups or substituents within a chemical molecule is mesomeric effects. It is defined as the polarity of a molecule formed by the interaction of two pi bonds or two pi bonds and lone electron pairs on a nearby atom. The effect is defined in terms of electron-withdrawing behaviour and is discussed qualitatively. The symbol “M” is used to denote the mesomeric effect.
Mesomeric effects are negative (-M) for substituents belonging to electron-drawing groups and positive (+M) for substituents belonging to electron-donating groups.
FAQs on Mesomeric Effect and Its Examples for JEE
1. Why is mesomeric effect stronger than inductive effect? Is the mesomeric effect dominant over the inductive effect?
The stability of the carbocation increases as the resonance increases. The major factors for the decrease in electron deficiency and the rise in stability are the mobility of the charge and the number of resonance structures. In addition, the resonance effect is always more powerful and dominating than the inductive effect. Therefore, the mesomeric effect is stronger than the inductive effect.
Yes, the mesomeric effect is thought to be stronger and more dominant than the inductive effect in most substituents.
2. What are the applications of the mesomeric effect?
Stability of carbocation, stability of carbanion, strength of acid and bases and stability of free radicals are the applications of mesomeric effect.
Stability of carbocation: Stability of carbocation increases on resonance, therefore aromatic compounds are more stable than non aromatic compounds due to resonance.
Stability of carbanion: Stability of carbocation also increases on resonance.
Stability of free radicals: Stability of free radicals increases on resonance.
Strength of acid and bases: Acidity is directly proportional to -M effect and inversely proportional to +M effect.
The +M effect has a direct relationship with basicity, while the -M effect has an inverse relationship.
























