




What is the Modern Periodic Table?
The Periodic table of elements is one of the most important concepts in chemistry which helps to learn the periodic trends, physical and chemical characteristics of elements. This classification is based on the periodic variation in the electronic configuration of atoms. The modern periodic table is developed on the basis of the Modern Periodic Law which states that,
“The physical and chemical properties of the elements are periodic functions of their atomic numbers.”
When 94 naturally occurring elements were arranged in an ascending order of their atomic numbers, it was observed that elements having similar properties were repeated after regular intervals. In the long form of the modern periodic table, the horizontal rows are called periods and the vertical columns are called groups.
Long Form of Periodic Table
The long form of periodic table of elements is an arrangement of 144 elements (which also includes theoretical elements which are predicted but yet to be discovered) into periods and groups. The first 94 elements in the periodic table are naturally occurring and the rest are synthesised in the laboratory. The periodic table chart in Fig.1 gives a glimpse of the arrangements of elements in the periodic table in periods and groups. The periodic table element list in the below given chart will be helpful to study the nature of elements and their chemistry.
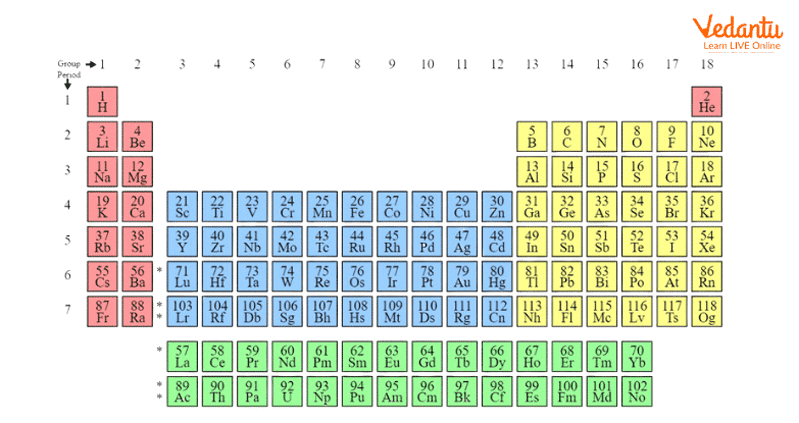
Modern Periodic Table with names of elements
The groups have elements with similar electronic configuration in their valence shell so they exhibit similar chemical properties. There are a total of 18 groups in the modern periodic table.
The elements are arranged in 7 periods in the periodic table chart. The period number indicates the highest principal quantum number (n) of the elements in that particular period. The first period has 2 elements and the rest periods have 8, 8, 18, 18 and 32 elements, respectively. The 14 elements of both sixth and seventh period are positioned in the two bottom rows in the periodic table. They are called lanthanoids and actinoids respectively.
Elemental Electronic Configuration-Periods
The consecutive periods in the Periodic Table are connected with the filling of the next higher principal energy level.
In the first period, there are two elements whose valence shell has the lowest principal energy level i.e., n=1. The elements in the first period are hydrogen (1s1) and helium (1s2).
In the second period, (n=2), 2s and 2p orbitals are filled. For example, the first element in the second period is lithium (Z=3) and here the third electron enters the 2s orbital. Second period has 8 elements.
The third period (n=3) has 8 elements and valence electrons are present in 3s and 3p orbitals.
The fourth period (n=4) starts at potassium, where the valence electron fills the 4s orbital. Next 3d orbitals are filled since they are energetically favorable than 4p. Such elements are called 3d transition elements. Then 4p orbitals are filled and hence the fourth period has 18 elements.
The fifth period (n=5), 4d the transition series starts at yttrium $Z=39$.This period ends with the filling up of the 5p orbitals. It also has 18 elements.
The sixth period (n=16) has 32 elements and here the electrons fill 6s, 4f, 5d, and 6p orbitals. The elements with valence electrons filling the 4f orbitals are called the lanthanide series.
The seventh period (n=7) element electrons fill in 7s, 5f, 6d, and 7p orbitals and they contain the man-made radioactive elements. The filling up of the 5f orbitals give rise to the 5f-inner transition series known as the actinide series.
Elemental Electronic Configuration-Groups
Elements in a group have similar valence shell electronic distribution and hence similar chemical properties. The Group 1 elements are also called alkali metals and have ns1 valence shell electronic configuration. For example, Lithium (Z=1) has 1s22s1 valence shell electronic configuration. The elements are further classified into s-block, p-block, d-block, and f-block depending on the orbitals in which valence electrons are filled. The two exceptions are Hydrogen and Helium.
The s-Block Elements
Group 1 (alkali metals) and Group 2 (alkaline earth metals) elements which have ns1 and ns2 valence shell electronic configurations are known as s-Block elements. They lose the outermost electron to form 1+ ion or 2+ ion for alkali and alkaline metals, respectively. They are thus reactive with low ionisation enthalpies. As we go down the group, the reactivity and metallic character increases.
The p-Block Elements
The p-Block Elements consist of elements of groups 13 to 18. The outer shell configuration varies from ns2np1 to ns2np6 in each period. All the last period elements are noble elements and its outer orbitals are completely filled by electrons. They have very low reactivity. The group 16 (Chalcogens) and group 17 (halogens) have high electron gain enthalpies and can add one or two electrons to attain a stable outermost configuration.
The d-Block Elements (Transition Elements)
The d-block elements consist of Group 3 to 12 in the Periodic Table. These elements are characterised by the filling of inner d orbitals by electrons. The general outer electronic configuration of the d-block is $(n-1)d^{1-10}ns^{0-2}$. They are all metals and form coloured ions. They exhibit variable oxidation states and paramagnetism. However, Zinc, Cadmium, and Mercury have electronic configuration, $(n-1)d^{10}10s^2$ and they do not behave like transition elements.
The f-Block Elements (Inner-Transition Elements)
The last two row elements down of the periodic table are called Lanthanoids and Actinoids and have valence shell electronic configuration$(n-2)f^{1-14}(n-1)d^{0-1}s^2$. The last electron is filled in the f-orbital. They are all metals and in each series the properties of the elements are similar. The actinides can have a large number of oxidation states. Hence their chemistry is complicated. These elements are radioactive.
Mendeleev’s Periodic Table
Dimitri Mendeleev is known as the father of the periodic table. He developed the first iteration of the periodic table. Mendeleev’s periodic table is based on Periodic law which states that on arranging elements in the increasing order of their atomic weights, the elements will show similar physical and chemical properties at regular intervals. Mendeleev’s Periodic Table was published in 1869. When Mendeleev developed his Periodic Table, scientists didn't know about the internal structure of atoms and even then, he was able to predict the properties of the elements.
Summary
The Periodic Table of elements with real names helps to understand the chemistry behind the various elements by arranging them in a periodic fashion of similar chemical and physical properties. The first periodic table developed by Mendeleev was based on atomic masses.
In the Modern Periodic Table, the elements are arranged in the order of their atomic numbers in seven horizontal rows (periods) and eighteen vertical columns (groups). The elements in the same group have similar chemical properties, as their valence electronic configuration is similar. Based on the electronic configuration, elements in the periodic table are divided into s-block, p-block, d-block, and f-block elements.
FAQs on Modern Periodic Table - A Collection of Chemical and Physical Properties of Elements for JEE
1. How is the modern periodic table arranged?
The elements in the modern periodic table are arranged in increasing order of atomic number. The elements are then divided into rows (Periods) and columns (groups). There are 7 periods in the table and elements are arranged in increasing order of atomic number. The elements in the same groups have similar valence shell electronic configurations and show similar chemical properties. There are 18 groups in the modern periodic table.
The metals are found on the left side of the periodic table, and the non-metal elements are found on the right. An imaginary zig-zag line, starting at B-Al-Si, separates metals from nonmetals.
2. Why is hydrogen placed in the first group?
Hydrogen (Z=1) has only one s-electron and hence placed in the first group (Alkali metal group). Like alkali metals it can lose one electron to form hydrogen ions. Similar to alkali metals, hydrogen combines with non-metals such as oxygen and sulphur and forms their oxides and sulphides. Similar to alkali metals, hydrogen also acts as a strong reducing agent. It can also gain one electron to achieve a stable arrangement and behaves similar to the halogen family (group 17).
Like alkali metals, it shows an oxidation state of -1. Similar to halogens, hydrogen easily combines with nonmetals such as carbon, silicon, nitrogen, etc to form covalent compounds.
























