




How Does Surface Energy Affect Real-World Materials?
Surface Energy is a critical property in physics, describing the energy required to create a unit surface area of a material. This concept appears in many JEE Physics questions, especially those about solid surfaces and their interactions with liquids.
Surface Energy Definition
Surface energy is the reversible work needed to create one square meter of surface at constant temperature and pressure. Unlike bulk energy, it relates to atoms at the material's surface which are less tightly bound than those inside.
Surface Energy vs Surface Tension
In solids, surface energy and surface tension differ: surface energy is work per area, while surface tension is force per unit length in liquids. However, for liquids, numerical values coincide since the surface is always in motion.
Surface tension is often confused with surface energy, but remember that for solids, surface energy also accounts for elastic or plastic deformation, which distinguishes it from liquids. For more details on this relation, see Surface Tension and Contact Angle.
Surface Energy Formula and Units
The general surface energy formula is:
| Quantity | Formula | Unit |
|---|---|---|
| Surface Energy (γ) | γ = Work / Area | J/m2 or N/m |
Surface energy units include Joule per square meter (J/m²) or Newton per meter (N/m). In exam questions, always confirm the units match the context, as mismatches can cause errors.
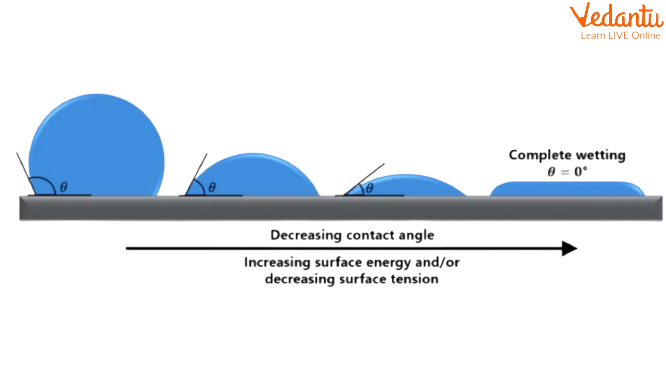
Measurement of Surface Energy
Surface energy measurement is challenging in solids due to surface contaminants and difficulties in isolating pure surfaces. Methods typically use contact angle measurements with probe liquids, relying on theories like Zisman or Fowkes models.
A common misconception is that surface energy can be measured as easily as in liquids; in reality, solid surfaces interact with environmental molecules, altering the true value.
These methods help determine the surface energy balance, crucial for understanding wetting, adhesion, and material compatibility in engineering and biology. For additional context, you can explore Capillary Action Explained.
Factors Affecting Surface Energy
Surface energy depends on atomic bonding strength, surface structure, and chemical composition. Solids like metals and ceramics have high surface energies, while polymers exhibit lower surface energies due to weaker intermolecular forces.
A micro-example: Water on clean glass spreads, indicating high surface energy, but on a waxed surface, water beads up due to low surface energy.
Surface Energy in Materials
Different materials show a wide range of surface energies. Metals may reach hundreds to thousands of mJ/m², while surface energy of plastics like PTFE is much lower, explaining their non-stick behavior.
The surface energy of water is especially high due to hydrogen bonding, playing a fundamental role in capillarity and droplet formation, key topics for JEE preparation. To understand how these properties influence solids, check Properties of Solids on Vedantu.
Applications and Implications
Understanding surface energy helps in designing superhydrophobic materials, improving adhesion in composites, and optimizing wettability in biomedical devices. In electronics, surface energy affects thin film fabrication and nanostructure growth.
- High surface energy: good wettability, strong adhesion
- Low surface energy: poor wettability, non-stick surfaces
- Surface energy balance impacts composite interfaces
A common mistake in JEE is to ignore the role of surface energy balance equation when explaining wettability, leading to incomplete answers.
Summary
Surface energy is fundamental to surface phenomena and material science, connecting microscopic atomic properties to macroscopic effects like wettability and adhesion. For a deeper dive, refer to the Second Law of Thermodynamics and its relationship with surface interactions, as covered on Vedantu.
FAQs on Understanding Surface Energy: Key Concepts and Applications
1. What is surface energy in physics?
Surface energy is the amount of energy required to increase the surface area of a liquid or solid by a unit amount.
Key points include:
- Surface energy is measured in Joules per square meter (J/m²).
- It arises due to unbalanced molecular forces at the surface of a material.
- It plays a crucial role in phenomena like wetting, capillarity, and cohesion.
2. Why do liquids possess surface energy?
Liquids possess surface energy because molecules at the surface experience a net inward force, making their energy higher than those inside the liquid.
Reasons include:
- Molecules at the surface are not fully surrounded by similar molecules, leading to imbalance in molecular attractions.
- This unbalanced force creates additional potential energy, known as surface energy.
- It is closely related to surface tension.
3. How is surface energy related to surface tension?
Surface energy and surface tension are closely related physical concepts, especially for liquids.
- Surface tension is defined as the force per unit length on the surface of a liquid.
- Surface energy is the work done to increase the surface area by one unit.
- For liquids, surface energy (γ) numerically equals surface tension at constant temperature.
4. What are some important applications of surface energy?
Surface energy has significant applications in both science and industry.
Key applications include:
- Wetting and adhesion of liquids on solids
- Detergent action and cleaning
- Formation of droplets and bubbles
- Capillary action in plants and tubes
- Coating and painting processes
5. What factors affect the surface energy of a material?
Surface energy is influenced by several factors.
Main factors:
- Nature of material (liquids, solids, polymers, metals)
- Temperature – typically, higher temperature leads to lower surface energy
- Presence of impurities or surfactants
- Surface roughness and treatment
- Chemical composition of the surface
6. Give the SI unit of surface energy.
The SI unit of surface energy is Joule per square metre (J/m²).
- It measures the energy needed to create one square metre of surface area.
- The same unit is used for both surface energy and surface tension in SI.
7. Describe an experiment to measure surface energy of a liquid.
Surface energy of a liquid can be measured using the drop weight or drop volume method.
Procedure:
- Form drops at the end of a thin tube using the liquid.
- Count the number of drops for a known volume.
- Using the Stalagmometer and known densities, calculate surface tension and thus surface energy.
8. What is the difference between surface energy and surface tension?
Surface energy and surface tension differ mainly in their definitions and units.
- Surface energy is energy per unit area (J/m²).
- Surface tension is force per unit length (N/m).
- For liquids, the numerical value of both quantities is the same at a given temperature.
9. What is the role of surface energy in wetting and adhesion?
Surface energy controls how well a liquid spreads (wets) and sticks (adheres) to a surface.
Role includes:
- High surface energy materials are more easily wetted and adhered to.
- Low surface energy materials resist wetting and adhesion.
- This principle is used for coatings, waterproofing, and printing technology.
10. Explain the significance of surface energy in everyday life.
Surface energy affects many daily phenomena.
Examples:
- Formation of rain droplets and dew
- Cleaning with soaps – lowering water's surface energy
- Adhesion of stickers
- Water-proofing of fabrics
























