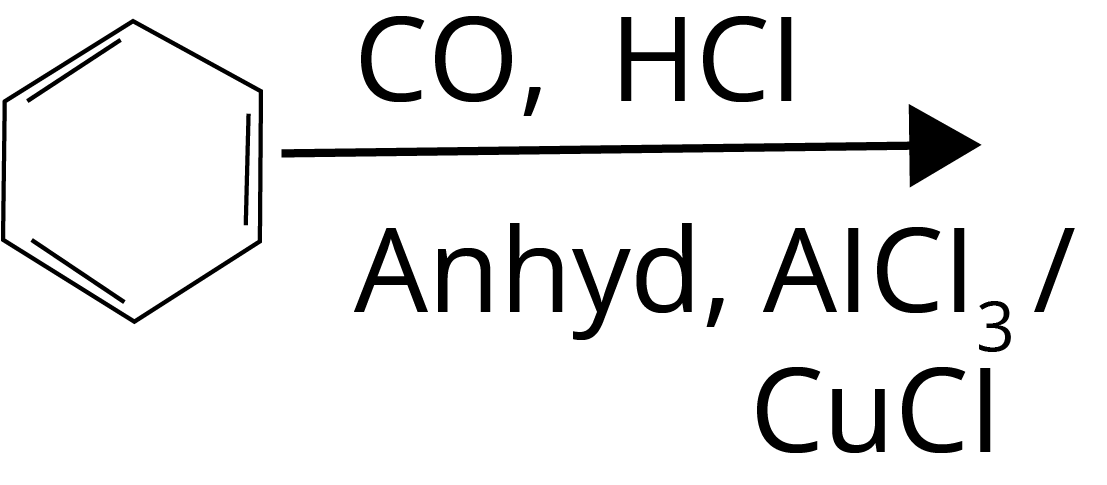Preparation of Alkanes
Alkanes are the class of hydrocarbons that are referred to as the saturated hydrocarbons, that is, hydrocarbons having all carbon atoms bonded to other carbon atoms or hydrogen atoms with single (sigma) bonds only. As the alkanes possess weak Van Der Waals forces, the first four members, C1 to C4 are gasses, C5 to C17 are liquids and those containing 18 carbon atoms or more are solids at 298 K. They are colourless and odourless. They are prepared in laboratories and industries through various techniques. Some techniques for the preparation of alkanes are discussed in this article.
Important Topics of Preparation, Properties and Reactions of Alkanes
Alkyl halide
Saturated Hydrocarbon
Halogenation
Hydrogenation
Pyrolysis
Decarboxylation
Wurtz Reaction
Substitution Reaction
Grignard Reagent
Important Definition of Preparation, Properties and Reactions of Alkanes
Physical Properties of Alkanes
Because of the covalent nature of C-C and C-H bonds and the small variation in electronegativity between carbon and hydrogen atoms, alkanes are nearly non-polar molecules.
Gases (C1 to C4), liquids (C5 to C17), and solids (having 18 carbon atoms or more) make up the first four members.
They have no colour and emit no odour.
With increasing molecular mass and surface area, the boiling point rises steadily.
Chemical Properties of Alkanes
Alkanes are generally non-reactive in nature due to their nonpolar nature. But, it gives some reactions. The chemical reactions of alkanes are given below:
1. Halogenation Reaction
CH4 + Cl2 → CH3Cl + HCl (in presence of sunlight)
CH3Cl + Cl2 +→ CH2Cl2 + HCl (in presence of sunlight)
CH2Cl2 + Cl2 → CHCl3 + HCl (in presence of sunlight)
CHCl3 + HCl → CCl4 + HCl (in presence of sunlight)
It is found that the rate of reaction of alkanes with halogens (X) is: F2 > Cl2 > Br2 > I2.
Rate of replacement of hydrogens (H) of alkanes (R) is: 3° > 2° > 1°.
2. Combustion
CH4 + 2O2 → CO2 + 2H2O + energy (Complete combustion)
CH4 + 2O2 → C + 2H2O (incomplete combustion)
3. Controlled Oxidation
2CH4 + O2 + Cu → CH3OH (at 523K and 100atm)
CH4 + O2 + Mo2O3 → HCHO + H2O
2C2H6 + 3O2 + (CH3COO)2Mn→ 2CH3COOH + 2H2O
Normally, alkanes resist oxidation, however, potassium permanganate can oxidise alkanes with a tertiary H atom to the equivalent alcohols.
(CH3)3C-H + KMnO4 → (CH3)3C-OH
4. Isomerisation: The reagent anhydrous AlCl3 / HCl is used to convert linear hydrocarbons into chain hydrocarbons with the same number of atoms. The chemical formula of hydrocarbon remains the same only it gets converted from linear isomer to chain isomer. For example, the reaction of n-hexane with reagent anhydrous AlCl3 / HCl is shown below in which two chain isomers of n-hexane are formed.

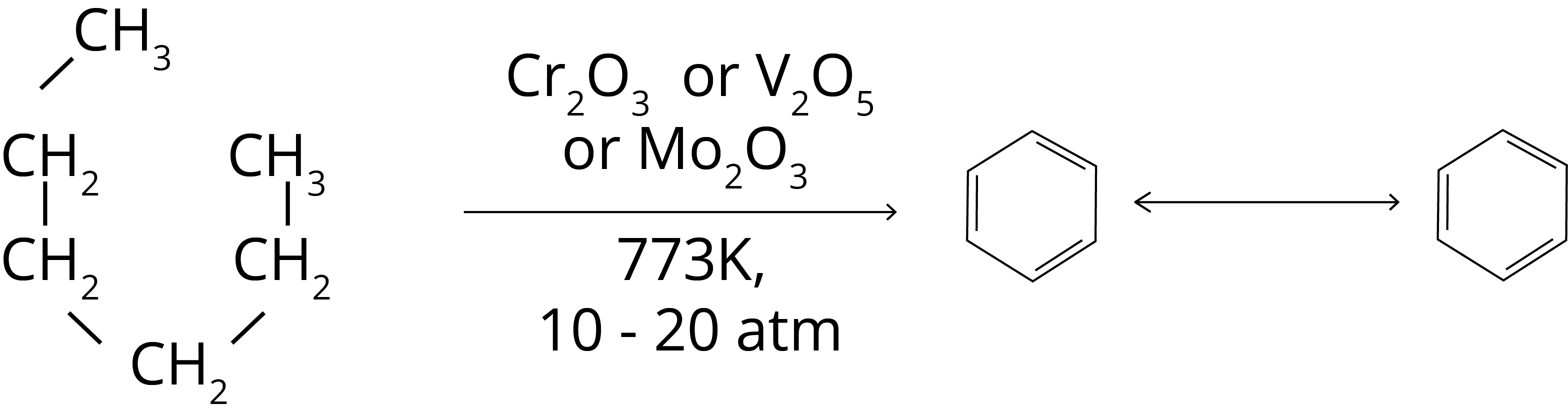
5. Aromatization: The aromatic hydrocarbons can be formed with linear hydrocarbons such as n-hexane with the help of d-block element’s oxides such as Cr2O3, V2O5 or MO2O3 at high temperature (773 K) and high pressure (10-20 atm). The reaction for the formation of benzene from n-Hexane is shown below.
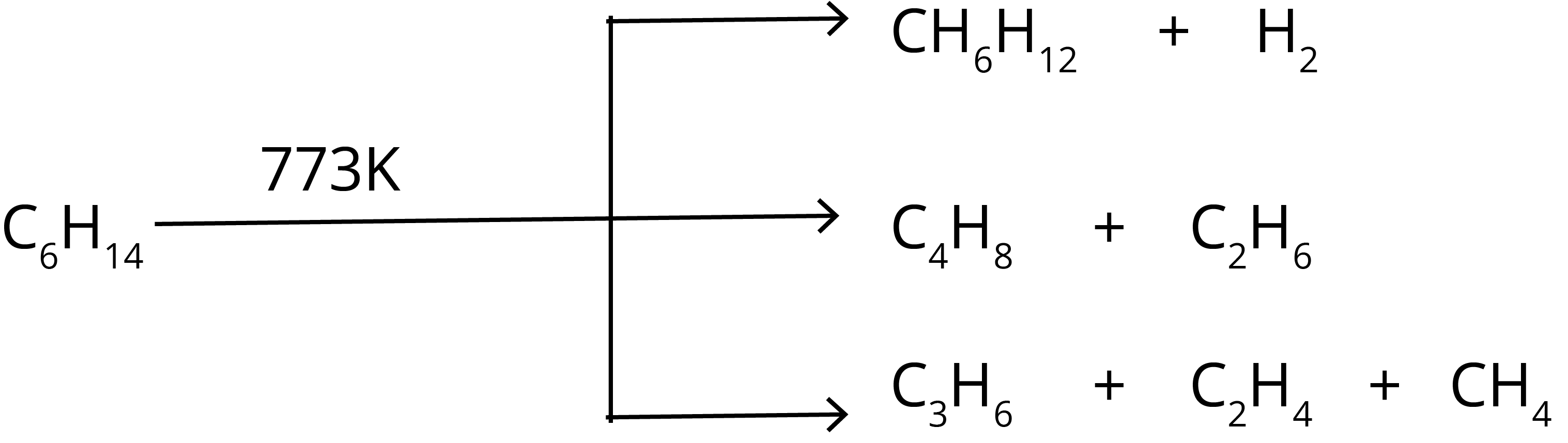
6. Pyrolysis: The name pyrolysis suggests that a reaction involves fire energy to break molecules. The linear hydrocarbons such as n-hexane at high temperature ( 773 K ) break into smaller hydrocarbons such as butane, propane, ethane, and methane. The chemical reaction of pyrolysis for n-hexane is shown below.
Preparation of Alkanes
There are several methods of preparation of alkanes. Some of the important methods are given below:
1. Hydrogenation: Hydrogen is added to unsaturated hydrocarbons to form saturated hydrocarbons.
$CH_{3}CH=CH_{2}+H_{2}\xrightarrow{\text{Pt/Pd/Ni}}CH_{3}-CH_{2}-CH_{3}$
$CH_{3}-C\equiv C-H+2H_{2}\xrightarrow{\text{pt/pd/Ni}}CH_{3}-CH_{2}-CH_{3}$
2. From Alkyl Halide: Hydrogen is added in place of halogen in this reaction.
R-Cl + H2 + Zn + H+ → R-H + HCl
3. Wurtz Reaction: Two hydrocarbons combine to form a hydrocarbon in the presence of sodium in dry ether.
2R-X + Na + dry ether → R-R + 2NaCl
4. Decarboxylation Reaction: Sodium salt of carbonic acid gets converted into hydrocarbon in the presence of sodium hydroxide and calcium oxide.
CH3COO-Na+ + NaOH + CaO → CH4 + Na2CO3
5. Kolbe’s Electrolytic Method: Sodium salt of carbonic acid gets converted into higher hydrocarbons with help of the electrolysis process.
CH3COO-Na+ + 2H2O → CH3-CH3 + 2CO2 + H2 + 2NaOH (on electrolysis)
Solved Examples Based on Preparation, Properties and Reactions of Alkanes
1. Sodium salt of which acid will be needed for the preparation of propane? Write a chemical equation for the reaction.
Ans: Sodium acid salt on decarboxylation gives alkanes. Butanoic acid sodium salt will be needed for the preparation of propane by a decarboxylation reaction.
CH3CH2CH2COO-Na+ + NaOH + CaO → CH3CH2CH3 + Na2CO3
Key Point: Sodium acid salt forms alkane by decarboxylation. The alkane produced in this reaction will have one carbon less from the parent sodium acid salt.
2. Write IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene in the absence of peroxide.
Ans: The addition of HBr to the alkene produces haloalkane. The haloalkane form will have halogen on the more alkylated carbon in the absence of peroxide. So, 2-Bromohexane will be produced in the given reaction.
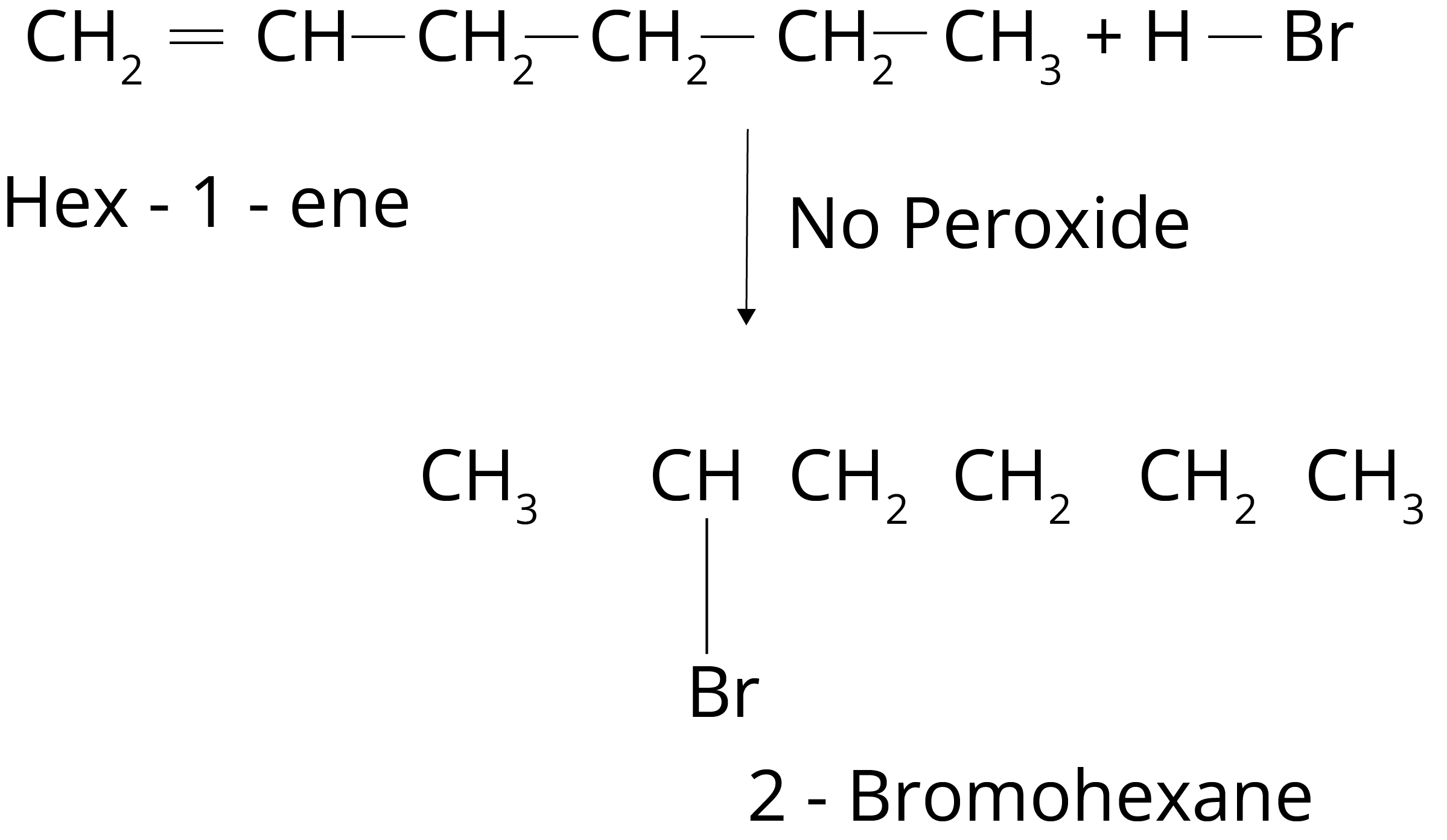
Key Point: In the absence of peroxide formation of haloalkane depends upon the stability of carbocation.
Solved Problems of Previous Years’ Question Based on Preparation, Properties and Reactions of Alkanes
1. The correct structure of 2,6-Dimethyl-dec-4-ene is :
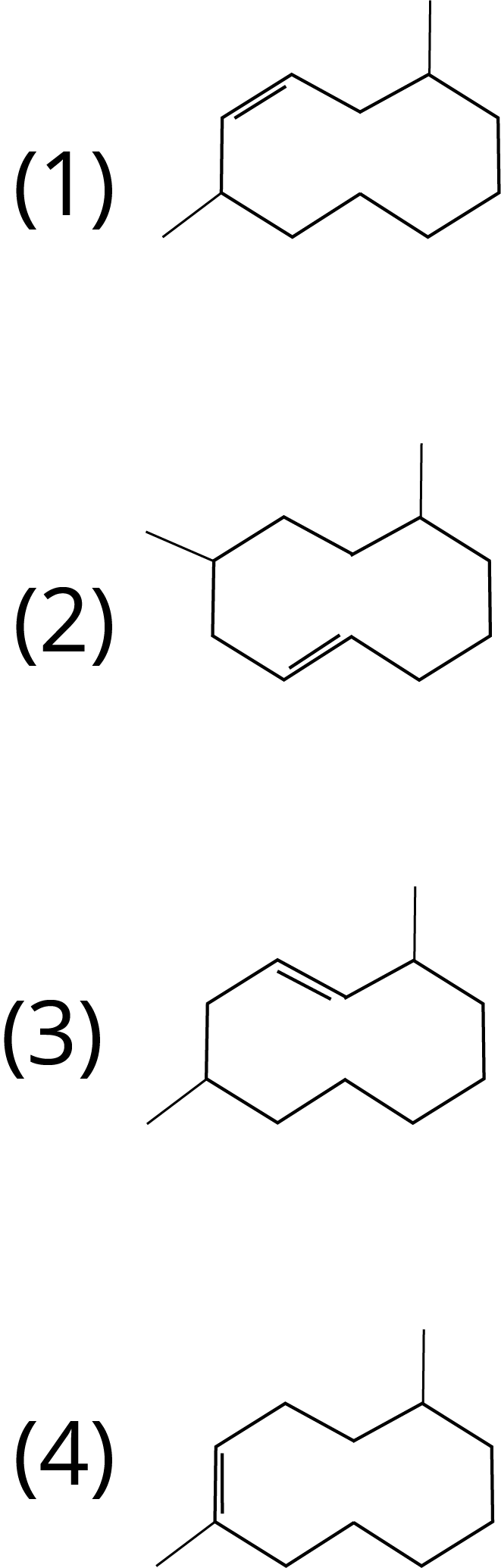
Ans: The correct answer for this question is option 1. Select the longest chain first, then give the lowest number to the double bond.
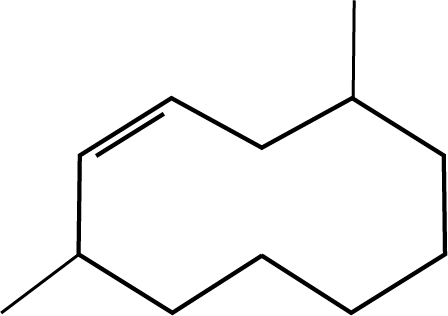
Trick: IUPAC naming includes the selection of the longest possible chain. The numbering should be done according to the lowest number rule. Also, give priority for the lowest number to the double bond, triple bond and functional group in the chain.
2. CH3CH2COO-Na+ + NaOH → CH3CH3 + Na2CO3
Consider the above reaction and identify the missing reagent/chemical.
a. B2H6
b. Red Phosphorus
c. CaO
d. DIBAL-H
Ans: The given reaction is a decarboxylation reaction. This reaction takes place in the presence of sodium hydroxide (NaOH) and lime (CaO).
Trick: The combination of NaOH and CaO is used in the proper fusion of the mixture that aids in the removal of the carbon dioxide from the reaction mixture.
3. Match List-I with List-II.
Choose the correct answer from the options given below.
(1) (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
(2) (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
(3) (a)-(i), (b)-(iv), (c)-(iii), (d)-(ii)
(4) (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
Ans: The correct answer for this question is the fourth option.
(a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
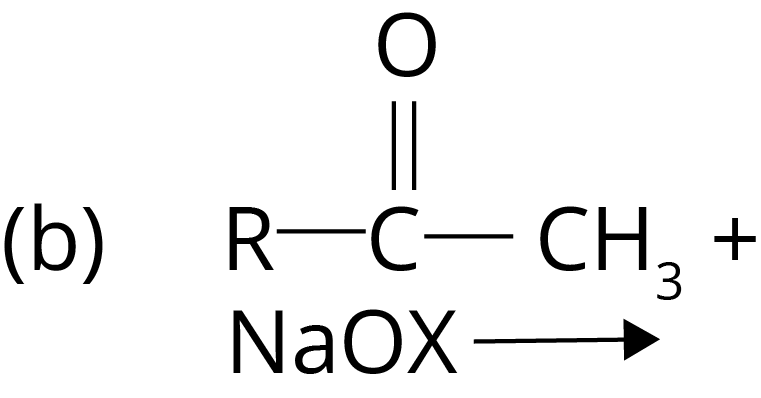
Trick: Esterification involves a reaction between alcohol and acid, Hell–Volhard–Zelinsky reaction takes place in the presence of red phosphorus and halogen, Gattermann-Koch reaction involves CO in the presence of HCl and lewis acid, and Haloform reaction takes place in the presence of Sodium oxide halogen salt.
Practice Questions
1. An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
(Ans: 3-Ethylpent2-ene)
2. Why is benzene extraordinarily stable though it contains three double Bonds?
(Ans: due to resonance)
Conclusion
In this article, we have provided important information regarding the chapter preparation, properties and reactions of alkanes such as important concepts, properties, reactions, etc. Students should work on more solved examples and previous years’ question papers for securing good grades in the JEE Advanced exams.
FAQs on JEE Important Chapter - Preparation of Alkanes
1. How significant is the Preparation, Properties, and Reactions of Alkanes chapter in the JEE-advance exam?
In the exam, around 1-2 questions from this chapter can be expected, carrying approximately 8 marks or about 2% of the total marks.
2. Does acid react with alkane?
Nonpolar alkane molecules do not react with ionic chemicals like most laboratory acids, bases, oxidising agents, or reducing agents because they are nonpolar.
3. Do alkanes have no reactivity?
Most reagents do not react with alkanes for two reasons. First, due to excellent orbital overlap, carbon carbon and carbon hydrogen single bonds are extremely strong. Second, because the electronegativity of both elements is fairly comparable, the carbon hydrogen bonds make alkane molecules neither acidic nor basic.