




Preparation, Properties and Reactions of Alkenes and Alkynes Notes
Carbon-carbon multiple bonds – double bonds, triple bonds, or both – are found in unsaturated hydrocarbons. Unsaturated hydrocarbons with at least one double bond are known as alkenes. Olefins are another name for alkenes (oil forming). The general formula for alkenes is CnH2n. Alkynes, like alkenes, are unsaturated hydrocarbons. At least one triple bond exists between two carbon atoms. In comparison to alkenes and alkanes, the number of hydrogen atoms in alkynes is still lower. Their general alkyne formula is CnH2n–2.
In this hydrocarbon notes for JEE Advance, we have discussed all the important concepts: alkyne formula, alkene formula, properties of alkenes and alkynes, preparation of alkynes, and reaction of alkenes and alkynes, that will be helpful in JEE Advanced examination.
Important Topics of Preparation, Properties and Reactions of Alkenes and Alkynes
Alkane
Alkene
Cyclic Haloalkane
Anti-Markovnikov's Rule
Pyrolysis
Decarboxylation
Wurtz Reaction
Markonikoff’s Rule
Aromatic Hydrocarbon
Important Definition of Preparation, Properties and Reactions of Hydrocarbons
Physical Properties of Alkenes
Physical properties of alkenes follow the same trend as the properties of alkanes.
Alkenes are similar to alkanes in terms of physical properties, with the exception of isomerism types and polar nature.
The first three members are gases, followed by fourteen liquids, and finally solids.
Ethene is a colourless gas with a sweet aroma. Colourless and odourless alkenes are the only ones that exist.
Alkenes are water insoluble but soluble in nonpolar solvents such as benzene and petroleum ether.
Except for aromatic hydrocarbons, they don't have much of an odour.
The boiling point rises continuously as molecule mass and surface area increase.
Chemical Properties of Alkenes
Because of their nonpolar nature, alkenes are often nonreactive. However, it elicits some responses. The reactions of alkenes are not the same as those of alkanes. The following are the chemical reactions of hydrocarbons:
1. Halogenation Reaction of Hydrocarbon
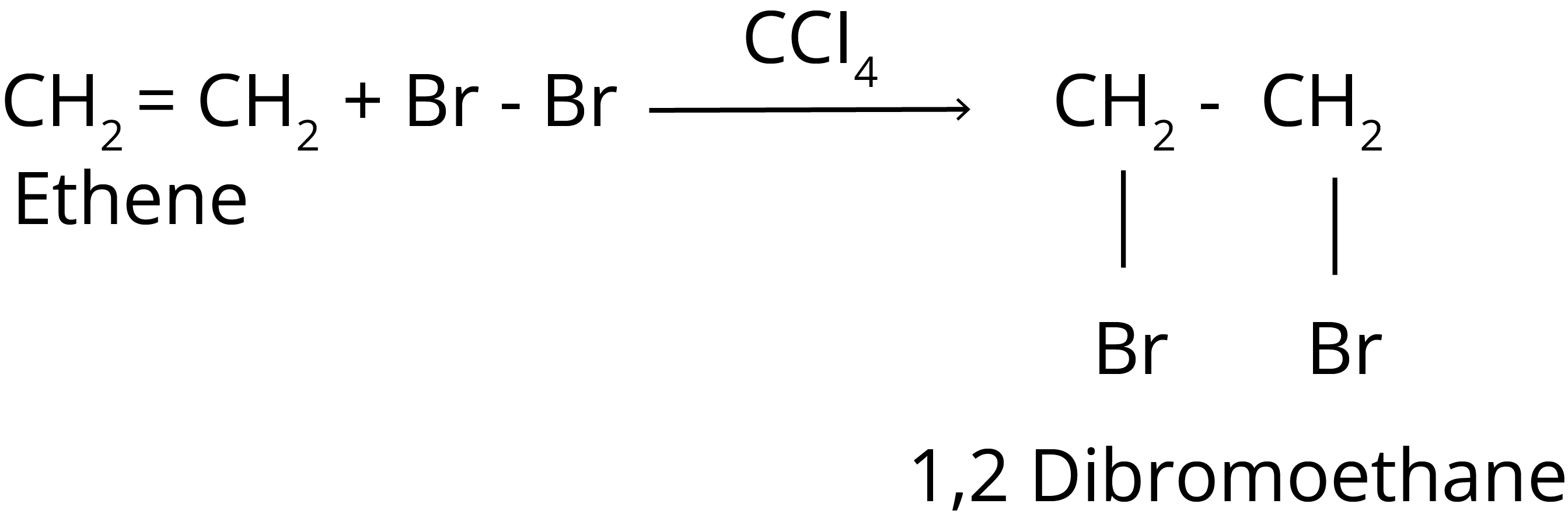
It is found that the rate of reaction of alkanes with halogens (X) is: F2 > Cl2 > Br2 > I2.
Rate of replacement of hydrogens (H) of alkanes (R) is: 3° > 2° > 1°.
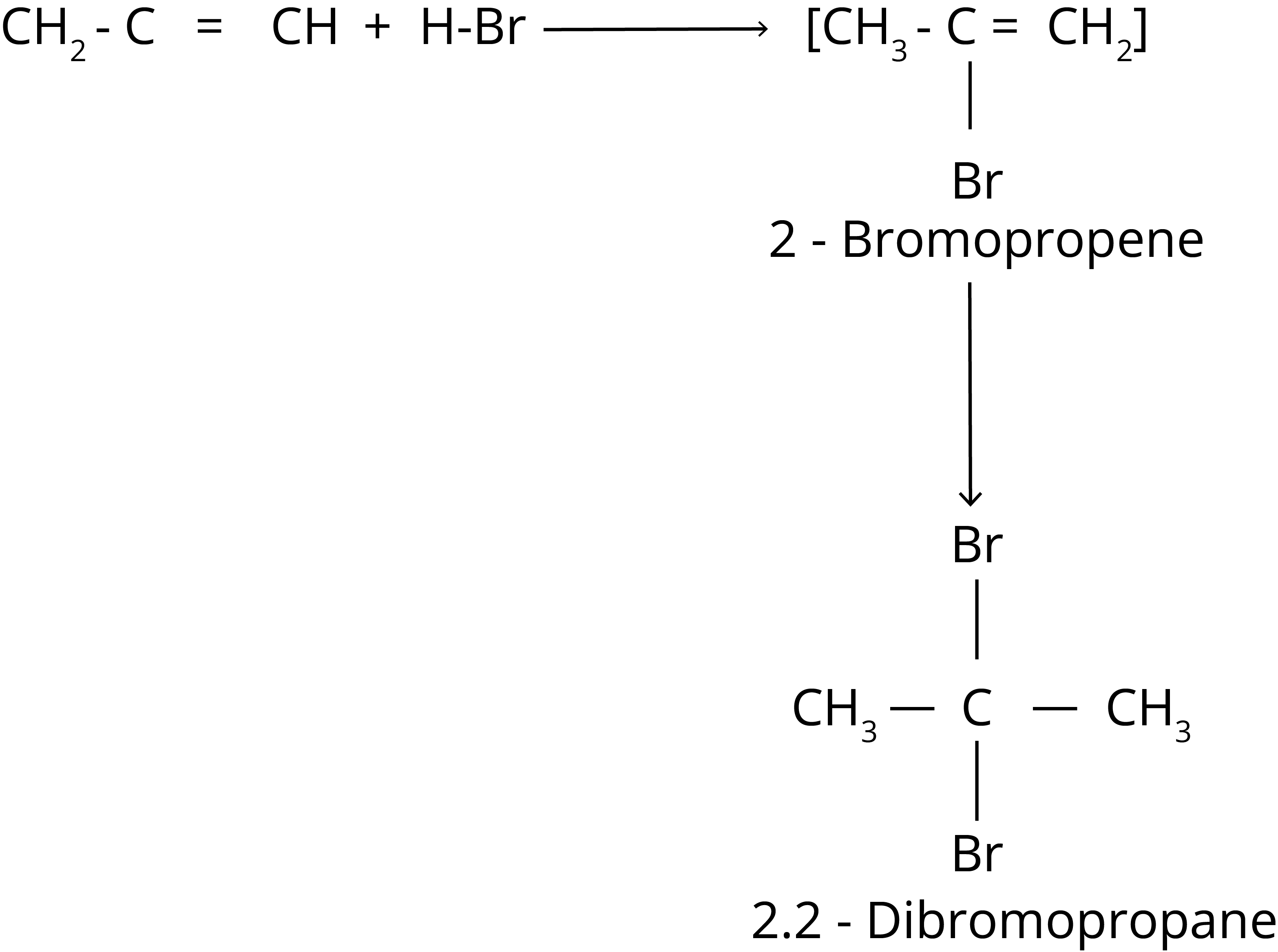
2. Addition of Hydrogen Halide
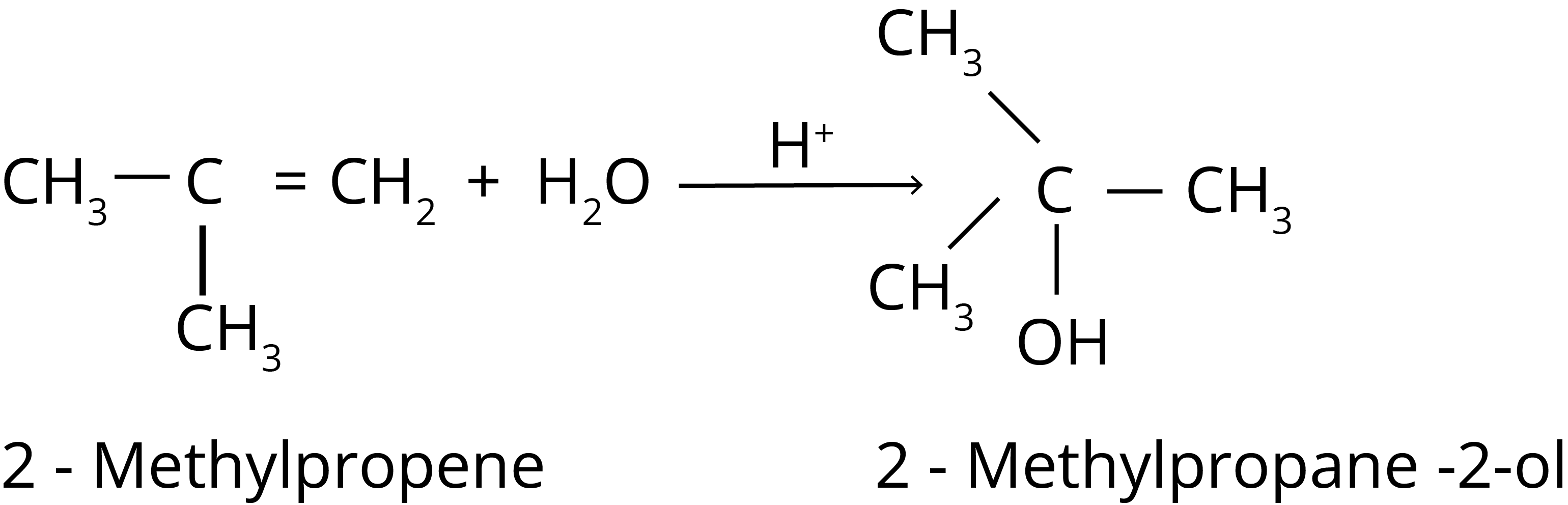
3. Addition of Water
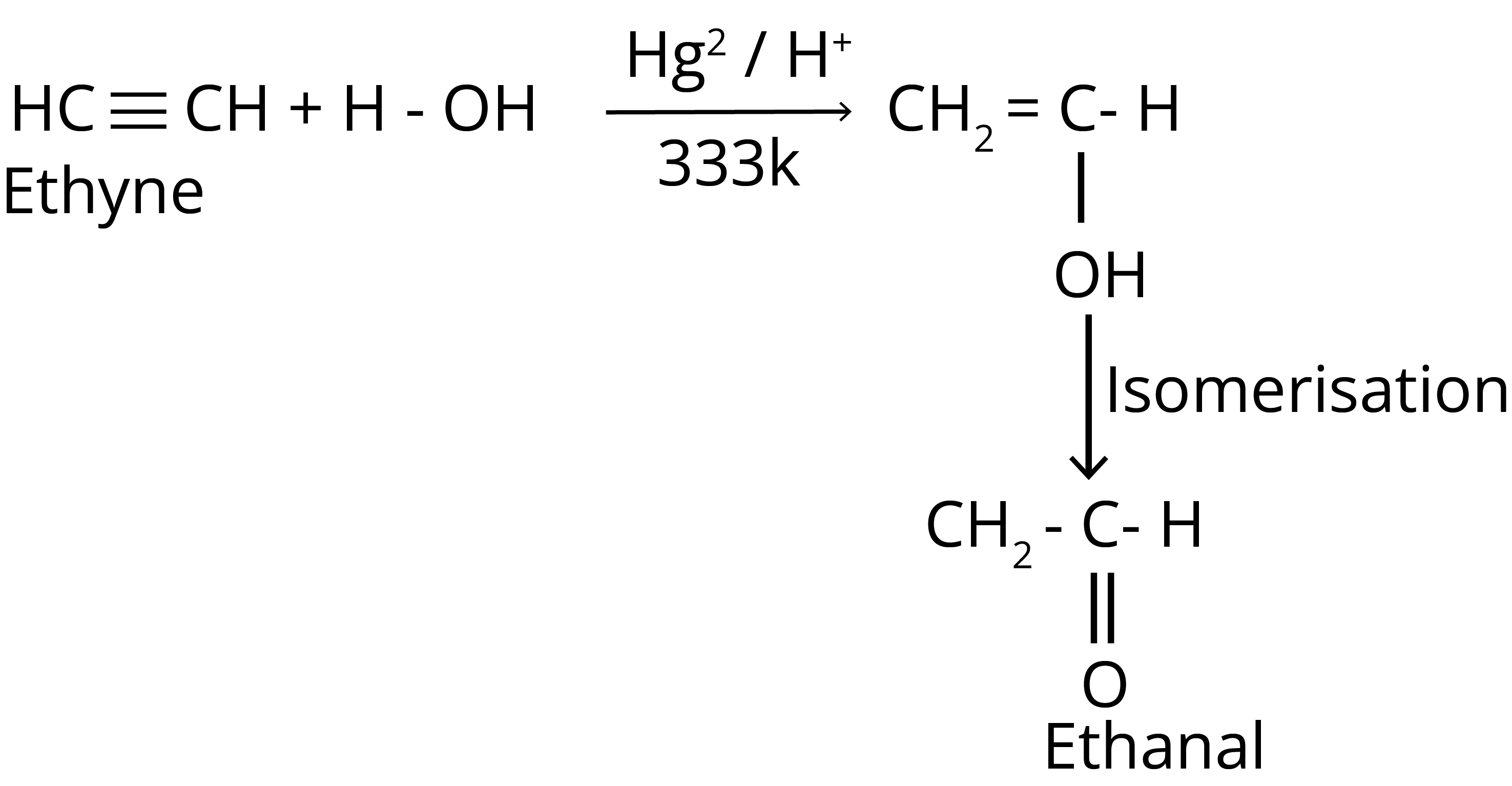
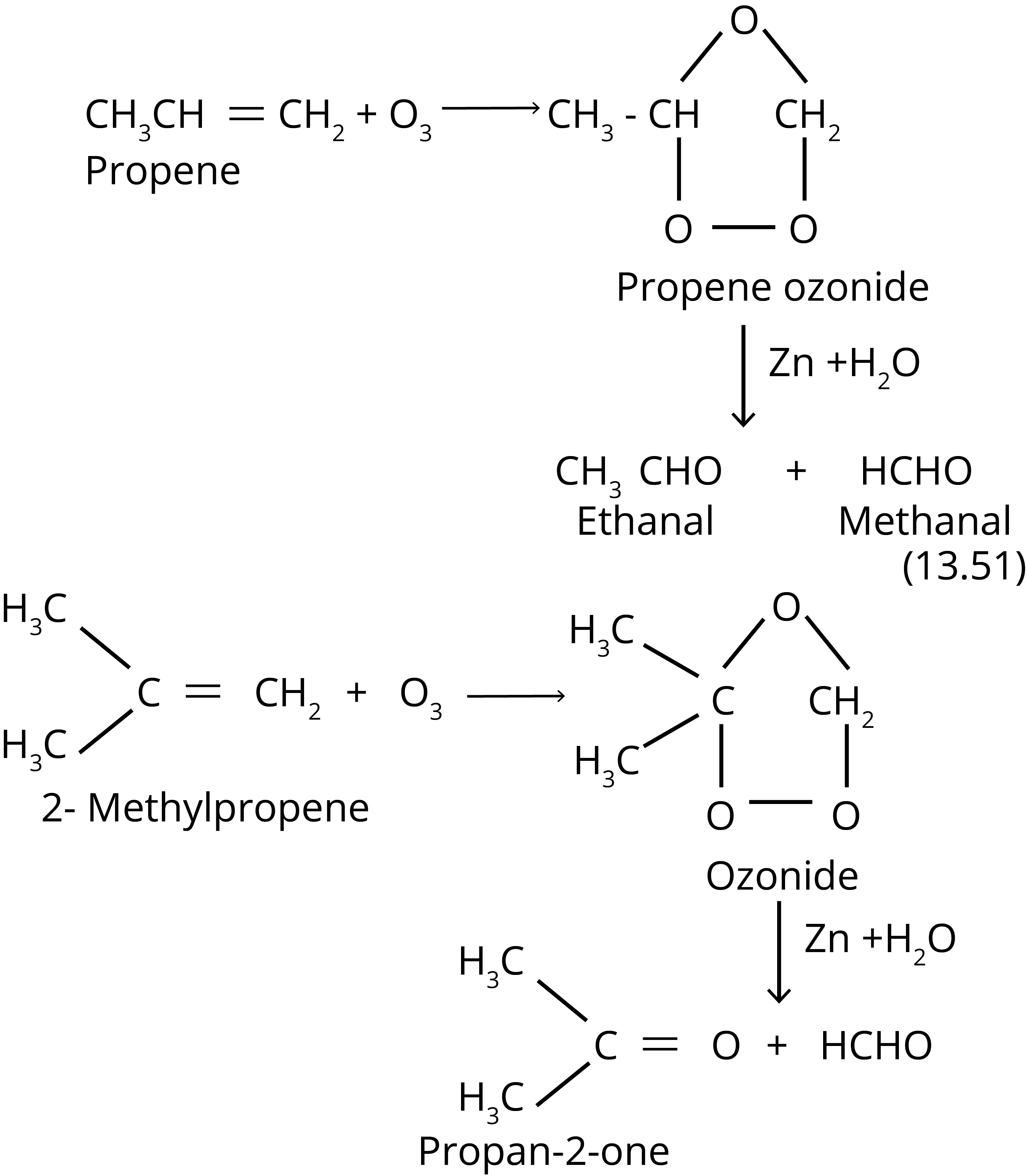
4. Ozonolysis
Markovnikov Rule
According to this rule, the negative component of the adding molecule is connected to the carbon atom with the fewest hydrogen atoms.
Mechanism of Markovnikov Rule
As demonstrated below, hydrogen bromide produces an electrophile, H+, which attacks the double bond to form a carbocation:
(image will be uploaded soon)
Because the secondary carbocation (b) is more stable than the main carbocation (a), the former takes precedence because it forms more quickly.
The carbocation (b) is attacked by Br- ion, resulting in the following product:
(image will be uploaded soon)
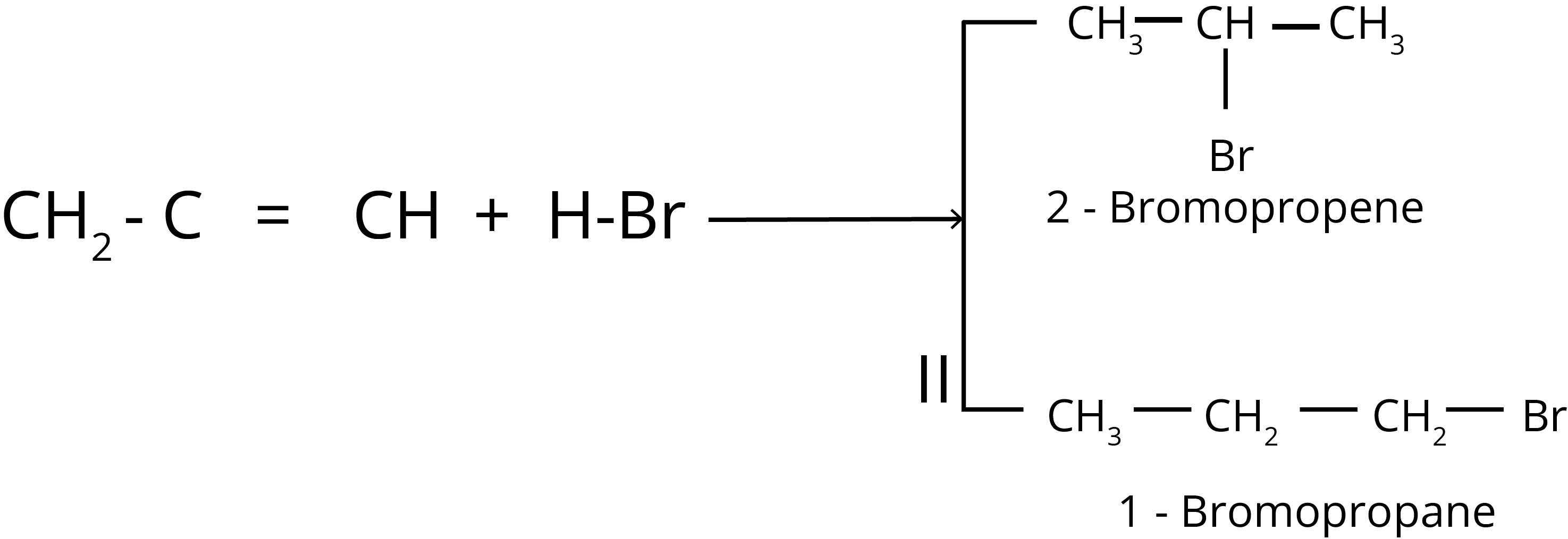
Anti-Markovnikov Addition or Peroxide Effect or Kharash Effect
The addition of HBr to unsymmetrical alkenes like propene goes against the Markovnikov rule in the presence of peroxide. This only happens with HBr and not with HCl or Hl.
Mechanism
As shown below, the peroxide effect is mediated through a free radical chain mechanism:
(image will be uploaded soon)
(image will be uploaded soon)
Physical Properties of Alkynes
Gases make up the first three members, liquids make up the next eight, and solids make up the last three.
Alkynes are all colourless.
The odour of ethylene is distinctive. Other members have no odour.
Alkynes are polar compounds with weak polarity. They are lighter than water and water insoluble, but soluble in organic solvents such as ethers, carbon tetrachloride, and benzene.
With increasing molar mass, their melting point, boiling temperature, and density rise.
Chemical Properties of Alkynes
Some of the common reactions of alkynes are: acidic nature, addition reactions and polymerisation reactions.
A. Acidic Character of Alkyne
(image will be uploaded soon)
B. Addition Reactions
(i) Addition of Hydrogen
(image will be uploaded soon)
(ii) Addition of Halogens
(image will be uploaded soon)
(iii) Addition of Hydrogen Halides
(image will be uploaded soon)
(iv) Addition of Water
(image will be uploaded soon)
(v) Cyclic Polymerisation
(image will be uploaded soon)
Preparation of Alkynes and Alkenes
1. From Alkynes
(image will be uploaded soon)
2. From Alkyl Halides
(image will be uploaded soon)
3. From Alcohols by Acidic Dehydration
(image will be uploaded soon)
4. From Calcium Carbide
$\text{CaC}{{\text{O}}_{\text{3}}}\xrightarrow{\Delta }\text{CaO}+\text{C}{{\text{O}}_{\text{2}}}$
$\text{CaO}+\text{3C}\to \text{Ca}{{\text{C}}_{\text{2}}}+\text{CO}$
$\text{Ca}{{\text{C}}_{\text{2}}}+\text{2}{{\text{H}}_{\text{2}}}\text{O}\to \text{Ca}{{\left( \text{OH} \right)}_{\text{2}}}+{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}$
5. From Vicinal Dihalides
(image will be uploaded soon)
Solved Examples Based on Chapter: Preparation, Properties and Reactions of Alkenes and Alkynes
1. Ethylene in reaction with bromine forms among the following products?
a) BrH2C-CH2Br
b) BrH2C=CH2Br
c) Br2HC=CHBr2
d) Br2HC-CHBr2
Ans: The correct answer is a. The above reaction between Ethene and bromine is known as an electrophilic halogenation reaction and the products usually formed are ethylene dihalides.
Key Point: The reaction is the addition reaction. The double bond of alkene is replaced by the addition of the bromine in the ethene to form a di-halo compound. This reaction is used to distinguish between alkane and alkyne.
2. An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ‘A’.
Ans: On ozonolysis, 'A' produces two moles of an aldehyde with a molar mass of 44 u. The presence of identical structural units on both sides of the double bond containing carbon atoms implies the creation of two moles of an aldehyde. As a result, the structure of 'A' can be written as XC = CX.
There are eight C-H bonds in total. As a result, 'A' contains 8 hydrogen atoms. There are three C-C bonds as well. As a result, the structure of 'A' has four carbon atoms. The structure of 'A' can be described as follows by combining the inferences:
(image will be uploaded soon)
Three C-C bonds, eight C-H bonds, and one C-C bond make up 'A.' As a result, But-2-ene is the IUPAC nomenclature for 'A.' Ozonolysis of 'A' occurs as follows:
(image will be uploaded soon)
The finished substance has a molecular mass of ethanal.
= {(2x12) + (4x1) + (1x16)}
= 44 u
Key Point: The product of ozonolysis can be simply predicted by replacing the double bond in alkene with double bond O (=O).
Solved Problems of Previous Year Question from the Chapter
1. Total number of hydroxyl groups present in a molecule of the major product P is
(image will be uploaded soon)
Ans: The correct answer for this question is 6.
(image will be uploaded soon)
Trick: Pd is a reducing agent, it adds hydrogen to the unsaturated bond. In the presence of potassium permanganate, all double bonds get replaced by hydroxyl groups.
2. The correct order of acid strength of the following carboxylic acids is :
(image will be uploaded soon)
Ans: The correct answer is 1.
(image will be uploaded soon)
Trick: Presence of double bond and triple bond increases the acidic character.
3. Choose the correct option(s) that give(s) an aromatic compound as the major product
(image will be uploaded soon)
Ans: The correct answer for this question is 1 and 2.
(image will be uploaded soon)
Trick: Alcoholic KOH leads to the dehydrohalogenation of the halogenated compound. Aromatization of thyme takes place in the presence of red hot iron.
Practice Questions
1. How do you account for the formation of ethane during the chlorination of methane?
Ans: In side reaction
2. Write the IUPAC names of the products obtained by the ozonolysis of Pent-2-ene.
Ans: Ethanal and propanal
Conclusion
In this alkene and alkyne note, we have provided important information regarding the chapter preparation properties and reactions of alkene and alkyne such as important concepts, properties, reactions, etc. Students should work on more solved examples and previous years’ question papers for securing good grades in the JEE advance exams.
FAQs on JEE Important Chapter - Preparation, Properties, and Reactions of Alkenes and Alkynes
1. What makes alkenes special?
Alkenes are nonpolar because they contain only carbon-carbon and carbon-hydrogen bonds and are not water soluble. They possess lower density than water (H2O).
2. Are alkenes completely saturated?
Unsaturated alkenes are used. This indicates that they have a double carbon bond. Due to the presence of only single bonds (C-C), the alkanes are saturated.
3. Is it possible to reduce alkenes?
Alkenes are reduced to alkanes by the addition of molecular hydrogen. The reaction is known as catalytic hydrogenation because it usually takes place in the presence of a transition metal catalyst.























