




What are Aromatic Hydrocarbons?
The class of substances known as 'aromatic compounds' was called after their pleasant odour. Arenes are another name for these hydrocarbons. The benzene ring was discovered in the majority of the compounds called Aromatic Hydrocarbons. Although the benzene ring is substantially unsaturated, the unsaturation of the benzene ring is preserved in the majority of aromatic compound reactions.
Important Topics of Reaction of Benzene
Kekulé structure
Aromatic Hydrocarbon
Halogenation
Hydrogenation
Resonance
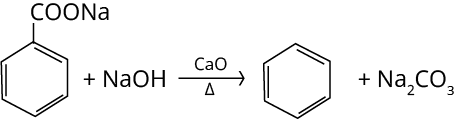
Decarboxylation
Toluene
Huckel Rule
Phenol
Important Definition of Reaction of Benzene
Physical Properties of Alkanes
Benzene and other aromatic molecules are nonpolar in nature.
It comes in the form of colourless liquids or solids with a distinct odour.
Aromatic hydrocarbons are water insoluble but readily miscible in organic solvents.
They emit a smoky blaze.
Chemical Properties of Benzene
Electrophilic substitution reactions characterise arenes. They can, however, perform addition and oxidation reactions under certain conditions. The chemical reactions of benzene are given below:
(a) Electrophilic Substitution Reactions
Nitration, halogenation, sulphonation, Friedel Crafts alkylation, and acylation reactions are frequent electrophilic substitution reactions of arenes in which the attacking reagent is an electrophile.
1. Nitration of Benzene
The nitration reaction of benzene is discussed below:
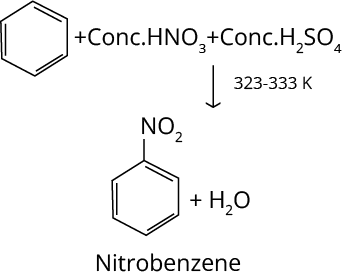
The product formed in the above reaction is called nitrobenzene. The nitrobenzene formula is C6H5NO2.
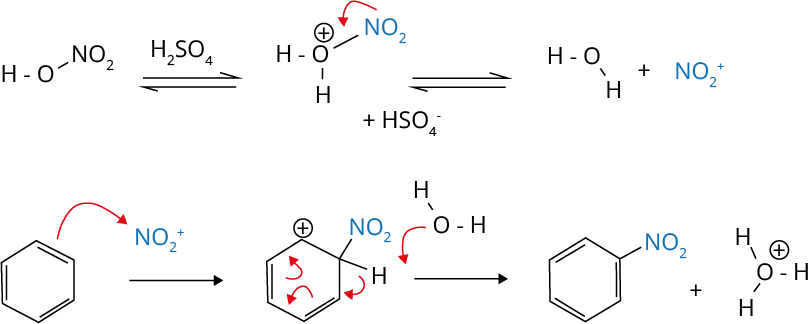
Mechanism of Nitration of Benzene
The mechanism for nitration of benzene is discussed below:
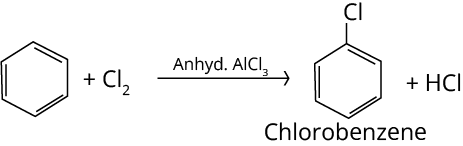
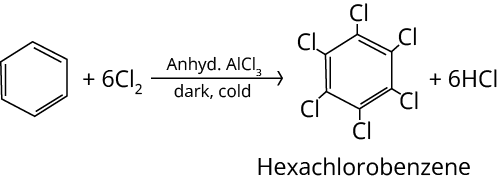
2. Halogenation
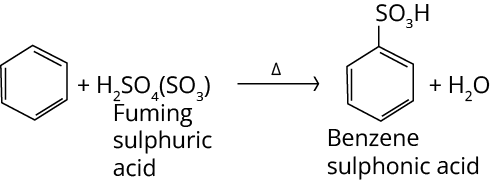
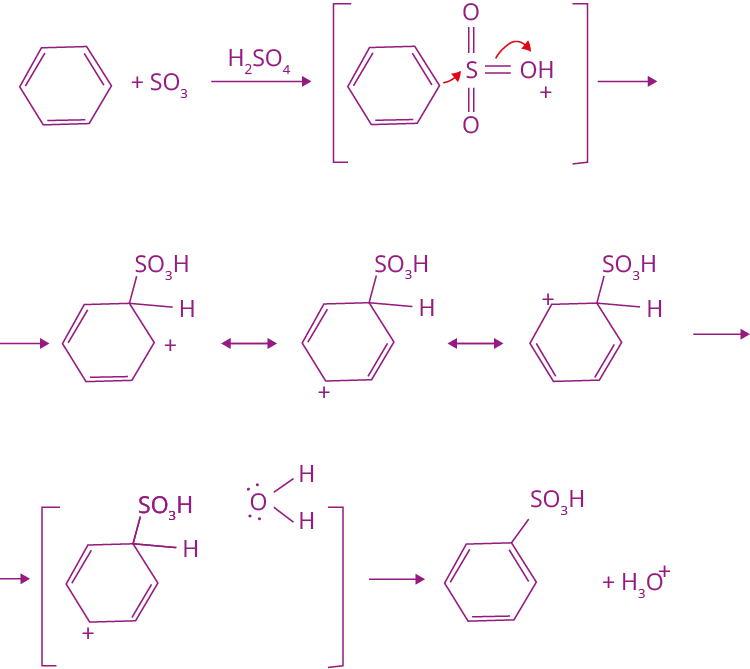
3. Sulphonation Reaction
Let us look at what is sulphonation? The addition of a sulphonic group to the benzene ring is known as sulphonation. The product formed is benzene sulphonic acid.
Mechanism of Sulphonation
The mechanism for sulphonation reaction is discussed below:
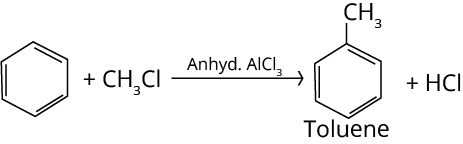
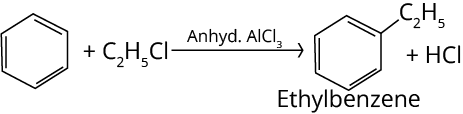
4. Friedel-Crafts Alkylation Reaction
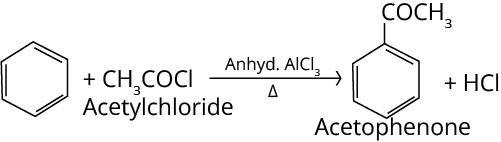
5. Friedel-Crafts Acylation Reaction
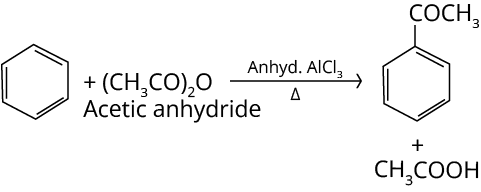
6. Reaction in Excess of Lewis Acid
7. Iodination of Benzene
The iodination of benzene (C6H6) rings takes place in the presence of an oxidising agent. The oxidizing agent is used to prevent the reduction of iodine as iodine gets readily reduced to I-.
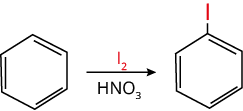
(b) Addition Reactions
1. Hydrogenation
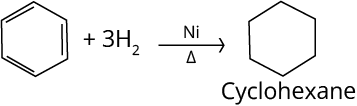
2. Halogenation
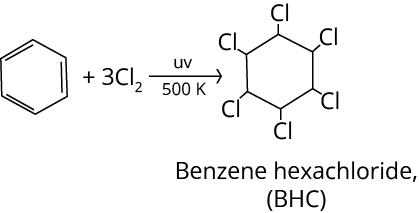
Preparation of Alkanes
There are several methods of preparation of benzene. Some of the important methods are given below:
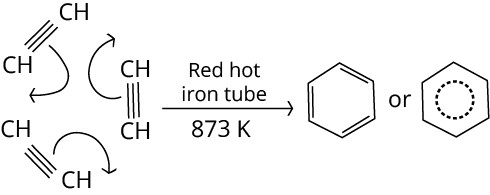
1. Cyclic Polymerisation of Ethyne
2. Decarboxylation of Aromatic Acids
Uses of Benzene
Benzene is a key component in the gaseous emissions of partially burned natural products, and it has a wide range of applications. It is a major component of gasoline and is widely used as an industrial chemical. Benzene is used in the production of plastics, synthetic fibres, rubber, paints and dyes, detergents, and many more products.
Some of the most important benzene uses and applications are given below.
Benzene is widely utilised as a solvent in industry. It's also employed for research and commercial applications. Many production companies use it as a solvent. It's employed in a variety of stages of the manufacturing process. It can also be used as a binding and absorbing agent.
Side-Effect of Benzene
In experimental animals such as rats and mice, benzene has been discovered to produce several forms of cancers when inhaled or eaten. These findings back up the discovery of an increased risk of leukaemia in humans. However, most human investigations have found no evidence of an increased risk of malignancies other than leukaemia in those who have been exposed to higher levels of radiation.
In the laboratory, benzene has been demonstrated to cause chromosomal alterations in bone marrow cells. (New blood cells are produced in the bone marrow.) In human leukemia cells, such alterations are prevalent.
Drowsiness, dizziness, headaches, tremors, confusion, and/or unconsciousness can result from inhaling excessive concentrations of benzene, which can cause drowsiness, dizziness, headaches, tremors, confusion, and/or unconsciousness. Vomiting, stomach irritation, dizziness, tiredness, convulsions, and a fast heart rate can all result from consuming foods or fluids contaminated with high quantities of benzene.
Inhaling or ingesting extremely high quantities of benzene can be fatal in severe situations. The skin, eyes, and throat might be irritated by benzene liquid or vapour. When skin is exposed to benzene, it can cause redness and blisters.
Solved Examples Based on the Chapter- Reaction of Benzene
1. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Ans: Benzene is a planar molecule with electrons delocalised above and below the ring plane. As a result, it has a lot of electrons. As a result, electron-deficient species, such as electrophiles, find it very appealing. As a result, it easily undergoes electrophilic substitution reactions.
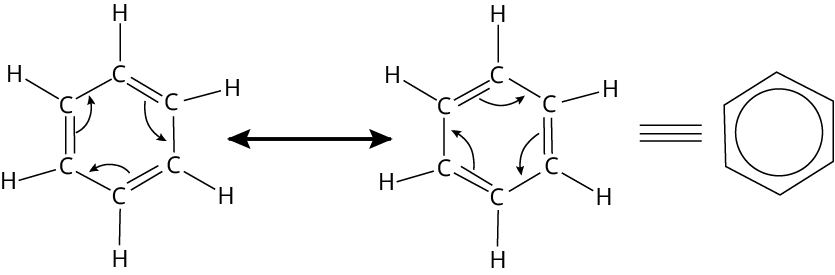
Key Point: Electrophilic substitution reaction takes place in electron deficient compounds. Benzene ring is an electron-rich compound due to the resonance mechanism.
2. How would you convert the ethylene into benzene?
Ans: Ethylene gets converted into a benzene ring in the presence of red hot iron.
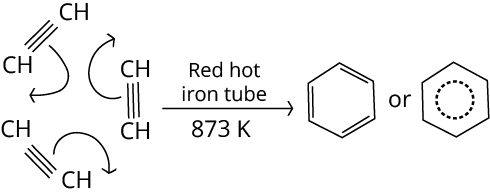
Key Point: Ethyne on passing through a red hot iron tube at 873K undergoes cyclic polymerization. Three molecules polymerise to form benzene.
Solved Problems of Previous Year Question - Reaction of Benzene
1. With respect to the compounds I-V, choose the correct statement(s).
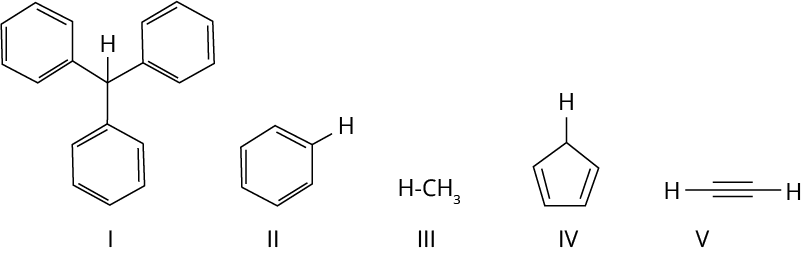
(A) The acidity of compound I is due to delocalisation in the conjugate base.
(B) The conjugate base of compound IV is aromatic.
(C) Compound II becomes more acidic, when it has a -NO2 substituent.
(D) The acidity of compounds follows the order I > IV > V > II > III.
Ans: The correct answer for this question is options A, B and C. In an option, the given hydrocarbon contains three benzene rings which withdraw electrons from attached carbon and in turn this hydrogen gets released.
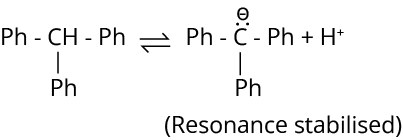
Electron withdrawing group increases the acidity of the benzene ring. After releasing one hydrogen compound, the fourth will become aromatic.
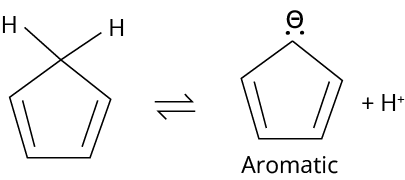
Trick: Acidic character increases with the presence of electron withdrawing group and aromaticity.
2. Newman projections P, Q, R and S are shown below:
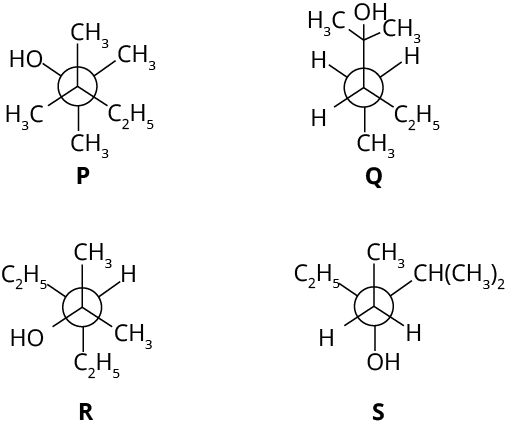
Which one of the following options represents identical molecules?
(A) P and Q
(B) Q and S
(C) Q and R
(D) R and S
Ans: The answer to the given question is Q and R.
Trick: For such questions, write the IUPAC name. The molecules that have the same name will have the same structure.
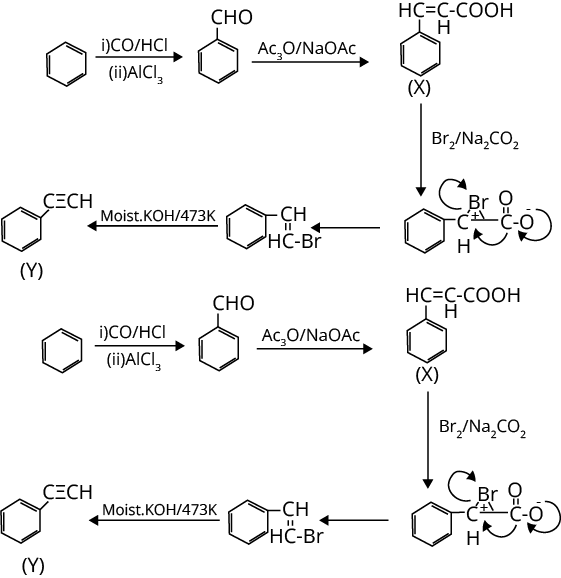
3. Treatment of benzene with CO/HCl in the presence of anhydrous AlCl3/CuCl followed by reaction with Ac2O/NaOAc gives compound X as the major product. Compound X upon reaction with Br2/Na2CO3, followed by heating at 473 K with moist KOH furnishes Y as the major product. Reaction of X with H2/Pd-C, followed by H3PO4 treatment gives Z as the major product. The compound Y is
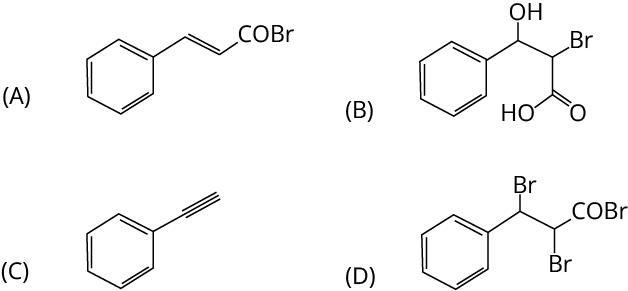
Ans: The correct answer is option C.
Practice Questions
1. How will you convert benzene into p-nitrobromobenzene?
Ans: By nitration
2. How will you convert benzene into acetophenone?
Ans: By acylation
Conclusion
In this article, we have provided important information regarding the chapter reaction of benzene such as important concepts, properties, reactions, etc. Students should work on more solved examples and previous years’ question papers for securing good grades in the JEE advanced exams.
FAQs on JEE Chapter - Reaction of Benzene
1. In the JEE advanced examination, how important is the reaction of the benzene chapter?
Nearly 1-2 questions arise in the exam from this chapter covering about 8 marks which makes about 2% of the total marks.
2. What are the main points to remember while tackling problems involving the reaction of benzene?
Students should practise writing the mechanism of the reaction for solving the questions from the JEE advanced chemistry reaction of benzene.
3. Are previous years’ question papers enough for scoring good marks in JEE advanced?
NCERT and previous years’ JEE advanced papers are adequate to score good marks in JEE Advanced exam. Solving previous 10 years JEE advanced papers gives us a significant edge because generally, 3-4 questions with the same concepts are sure to be repeated every year.























