
(A) In general phenolphthalein is used as an indicator for the titration of a weak acid (\[C{{H}_{3}}COOH\]) and strong base (\[NaOH\]).
(R) At the equivalence point solution is basic.
(A)- Both (R) and (A) are true and the reason is the correct explanation of assertion.
(B)- Both (R) and (A) are true but the reason is not the correct explanation of assertion.
(C) – Assertion (A) is true but reason (R) is false
(D)- Assertion (A) is false but reason (R) is true
Answer
233.1k+ views
Hint: At the equivalence point, the solution turns from colourless to pale pink colour.
The equivalence point is that point of the acid-base titration when the amount of the titrant added is sufficient to neutralize the analyte solution.
Phenolphthalein is a commonly used acid-base indicator and it has no colour in acidic conditions.
Complete step by step solution:
- In general, phenolphthalein is used as an indicator for the titration of a weak acid (\[C{{H}_{3}}COOH\]) and strong base (\[NaOH\]). This is because phenolphthalein changes its colour from colourless to pale pink at around a \[pH\]of 8 to 10 and the equivalence point for the titration of the weak acid and strong base lies around \[pH\]7 and upwards.
- Other indicators like methyl orange change its colour at around a \[pH\]of 4. So, there is a considerable difference between the \[pH\]of the colour change of the indicator and the equivalence point for the titration of the weak acid and strong base.
- So, phenolphthalein is the best indicator in this case.
The graph showing the equivalence point for the titration of the weak acid and strong base is as follows:
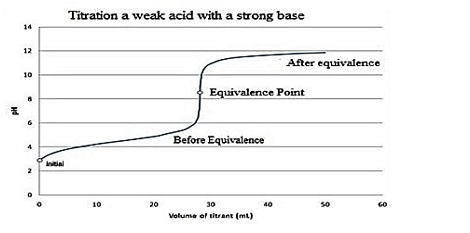
So, option B is the correct option.
Note: The reason behind the equivalence point for the titration of the weak acid and strong base lies around \[pH\]7 and upwards is due to the conjugate base of the weak acid. So, there will be a greater concentration of \[O{{H}^{-}}\]ions in the solution.
The equivalence point is that point of the acid-base titration when the amount of the titrant added is sufficient to neutralize the analyte solution.
Phenolphthalein is a commonly used acid-base indicator and it has no colour in acidic conditions.
Complete step by step solution:
- In general, phenolphthalein is used as an indicator for the titration of a weak acid (\[C{{H}_{3}}COOH\]) and strong base (\[NaOH\]). This is because phenolphthalein changes its colour from colourless to pale pink at around a \[pH\]of 8 to 10 and the equivalence point for the titration of the weak acid and strong base lies around \[pH\]7 and upwards.
- Other indicators like methyl orange change its colour at around a \[pH\]of 4. So, there is a considerable difference between the \[pH\]of the colour change of the indicator and the equivalence point for the titration of the weak acid and strong base.
- So, phenolphthalein is the best indicator in this case.
The graph showing the equivalence point for the titration of the weak acid and strong base is as follows:

So, option B is the correct option.
Note: The reason behind the equivalence point for the titration of the weak acid and strong base lies around \[pH\]7 and upwards is due to the conjugate base of the weak acid. So, there will be a greater concentration of \[O{{H}^{-}}\]ions in the solution.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

Square vs Rhombus: Key Differences Explained for Students

Power vs Exponent: Key Differences Explained for Students

Arithmetic Mean Formula Explained Simply

Algebraic Formula: Key Concepts & Easy Examples

Constants vs Variables: Key Differences Explained Simply

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

JEE Mains 2026 January 21 Shift 2 Question Paper with Solutions PDF - Complete Exam Analysis

JEE Main 2026 Jan 22 Shift 2 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

List of Highest T20 Scores in International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




