
Acetaldehyde reacts with ${\text{NaOH}}$ to form:
A. 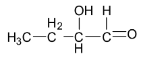
B. 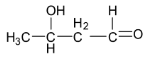
C. 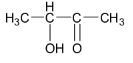
D. 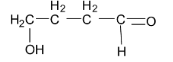
Answer
232.8k+ views
Hint: The carbon atom next to the carbonyl group in an aldehyde or a ketone is called ${{\alpha }}$ - carbon and the hydrogens attached to it are called ${{\alpha }}$ - hydrogens. These ${{\alpha }}$ - hydrogens are acidic due to the electron withdrawing inductive effect of the carbonyl group and so they can be easily abstracted by strong bases to give enolate ions.
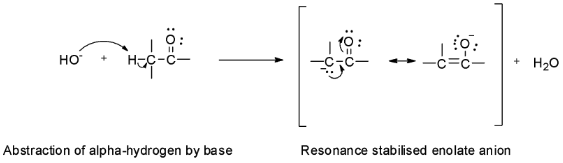
Based on this acidity of ${{\alpha }}$ - hydrogens, the aldol condensation reaction involves the reaction of aldehyde or ketone with dilute alkali to form a ${{\beta }}$ - hydroxyaldehyde or a ${{\beta }}$ - hydroxyketone. These ${{\beta }}$ - hydroxyaldehyde or ${{\beta }}$ - hydroxyketone are called aldols.
Complete step by step answer:
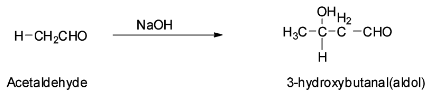
Acetaldehyde is an aldehyde containing ${{\alpha }}$ - hydrogen atoms and so they can be easily abstracted by a base. So acetaldehyde will undergo aldol condensation reaction with sodium hydroxide to give 3- hyroxybutanal which is an aldol. The reaction is shown below:
The mechanism of the above reaction is discussed below.
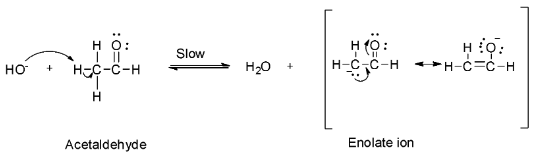
Step 1: Abstraction of acidic alpha hydrogen by the sodium hydroxide base to form an enolate ion.
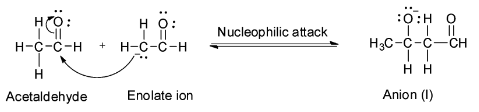
Step 2: Nucleophilic attack of the enolate on the second acetaldehyde molecule to form the anion (I).
Step 3: Abstraction of proton from water by the anion (I) to form aldol.

Option A is not correct as the given aldehyde structure contains hydroxy group in the alpha position and so it is not a betahydroxyaldehyde or aldol.
Option C is not correct as the given ketone structure contains hydroxy group in the alpha position and so it is not a betahydroxyketone or aldol.
Option D is not correct as the given aldehyde structure contains hydroxy group in the gamma position and so it is not a betahydroxyaldehyde or aldol.
Thus, option B is correct.
Note:
Aldehydes which do not have ${{\alpha }}$ - hydrogen atoms will not undergo aldol condensation reaction.
For example, formaldehyde does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give beta hydroxy aldehyde or aldol. Its structure is shown below.
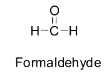
Pivaldehyde also does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give aldol. Its structure is shown below.
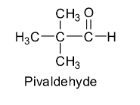

Based on this acidity of ${{\alpha }}$ - hydrogens, the aldol condensation reaction involves the reaction of aldehyde or ketone with dilute alkali to form a ${{\beta }}$ - hydroxyaldehyde or a ${{\beta }}$ - hydroxyketone. These ${{\beta }}$ - hydroxyaldehyde or ${{\beta }}$ - hydroxyketone are called aldols.
Complete step by step answer:
Acetaldehyde is an aldehyde containing ${{\alpha }}$ - hydrogen atoms and so they can be easily abstracted by a base. So acetaldehyde will undergo aldol condensation reaction with sodium hydroxide to give 3- hyroxybutanal which is an aldol. The reaction is shown below:

The mechanism of the above reaction is discussed below.
Step 1: Abstraction of acidic alpha hydrogen by the sodium hydroxide base to form an enolate ion.

Step 2: Nucleophilic attack of the enolate on the second acetaldehyde molecule to form the anion (I).

Step 3: Abstraction of proton from water by the anion (I) to form aldol.

Option A is not correct as the given aldehyde structure contains hydroxy group in the alpha position and so it is not a betahydroxyaldehyde or aldol.
Option C is not correct as the given ketone structure contains hydroxy group in the alpha position and so it is not a betahydroxyketone or aldol.
Option D is not correct as the given aldehyde structure contains hydroxy group in the gamma position and so it is not a betahydroxyaldehyde or aldol.
Thus, option B is correct.
Note:
Aldehydes which do not have ${{\alpha }}$ - hydrogen atoms will not undergo aldol condensation reaction.
For example, formaldehyde does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give beta hydroxy aldehyde or aldol. Its structure is shown below.

Pivaldehyde also does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give aldol. Its structure is shown below.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




