
Acetophenone and benzophenone can be distinguished by which of the following tests?
A.Knoevenagel reaction
B.Cannizzaro's reaction
C.Aldol condensation
D.HVZ reaction
Answer
233.1k+ views
Hint: For this question we need the reactants required for the above name reaction. Acetophenone has one benzene ring and benzophenone has two benzene rings. Both are ketonic in nature but the difference is one has alpha hydrogen and the other one does not have alpha hydrogen.
Complete step by step solution:
Aldehydes and ketones with at least 1 alpha hydrogen, when reacted with alkali, form beta hydroxy aldehyde or ketones respectively. The above reaction is known as Aldol condensation. The beta hydroxy aldehydes are called aldol and beta hydroxy ketones are called keto products.
Alpha hydrogen is that hydrogen which is directly attached to carbon next to the carbonyl group called alpha carbon. The structure of acetophenone is:
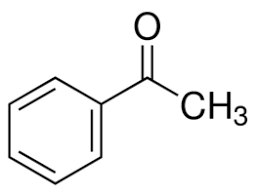
As we can see from the structure, the carbon on the right hand side of carbonyl has 3 alpha hydrogen’s directly attached to carbon next to carbonyl carbon. The left hand side has no hydrogen on adjacent carbon. So acetophenone will give an aldol test.
Now let us look at the structure of benzophenone:
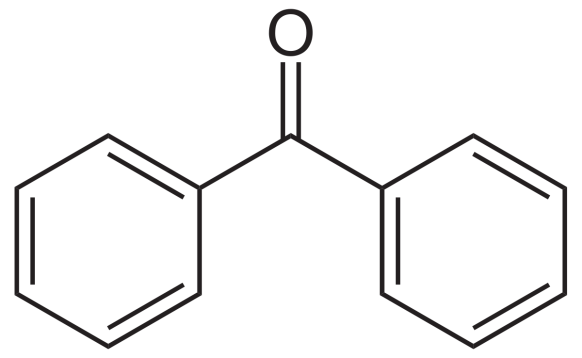
The carbon adjacent to carbonyl carbon (called alpha carbon) has no hydrogen atoms. Hence the above will not give the test for alcohol condensation. So we can distinguish acetophenone and benzophenone by aldol reaction.
So the correct option is C.
Note: In Knoevenagel reaction, the reactants are active methylene compounds whereas in cannizaro reaction, only one reactant is either aldehyde or ketone disproportionate. HVZ is the Hell Volhard Zelinsky reaction which has halogenated carboxylic acids as one of the reactants. So all these options stand eliminated.
Complete step by step solution:
Aldehydes and ketones with at least 1 alpha hydrogen, when reacted with alkali, form beta hydroxy aldehyde or ketones respectively. The above reaction is known as Aldol condensation. The beta hydroxy aldehydes are called aldol and beta hydroxy ketones are called keto products.
Alpha hydrogen is that hydrogen which is directly attached to carbon next to the carbonyl group called alpha carbon. The structure of acetophenone is:

As we can see from the structure, the carbon on the right hand side of carbonyl has 3 alpha hydrogen’s directly attached to carbon next to carbonyl carbon. The left hand side has no hydrogen on adjacent carbon. So acetophenone will give an aldol test.
Now let us look at the structure of benzophenone:

The carbon adjacent to carbonyl carbon (called alpha carbon) has no hydrogen atoms. Hence the above will not give the test for alcohol condensation. So we can distinguish acetophenone and benzophenone by aldol reaction.
So the correct option is C.
Note: In Knoevenagel reaction, the reactants are active methylene compounds whereas in cannizaro reaction, only one reactant is either aldehyde or ketone disproportionate. HVZ is the Hell Volhard Zelinsky reaction which has halogenated carboxylic acids as one of the reactants. So all these options stand eliminated.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




