Chemistry Notes for Chapter 10 Biomolecules Class 12 - FREE PDF Download
FAQs on Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26
1. What is the most effective sequence for revising Biomolecules in Class 12 Chemistry using summary notes?
Start your revision by understanding the classification of biomolecules into carbohydrates, proteins, lipids, and nucleic acids. Next, review the structure and types of carbohydrates (monosaccharides, disaccharides, polysaccharides), focusing on their functions and reactions. Then, move on to proteins, covering amino acid structure, peptide bonds, and the four levels of protein structure. Follow with lipids, looking at their types, structure, and saponification reactions. Finally, study nucleic acids, emphasizing DNA and RNA structures, replication, and biological roles. This sequence ensures logical flow and thorough coverage as per the CBSE 2025–26 syllabus.
2. How do concise revision notes improve retention and exam performance for Chapter 10?
Concise revision notes enhance retention by summarizing core concepts, key definitions, and essential formulas in an easy-to-review format. These notes save time, minimize information overload, and help students focus on high-weightage topics. By highlighting vital details and using diagrams or concept maps, students can quickly revisit important points before exams, leading to improved memory, understanding, and confidence while answering application-based questions.
3. Which core ideas and definitions should be prioritized when revising Biomolecules for Class 12 Chemistry?
Prioritize the following core ideas:
- Classification of biomolecules (carbohydrates, proteins, lipids, nucleic acids)
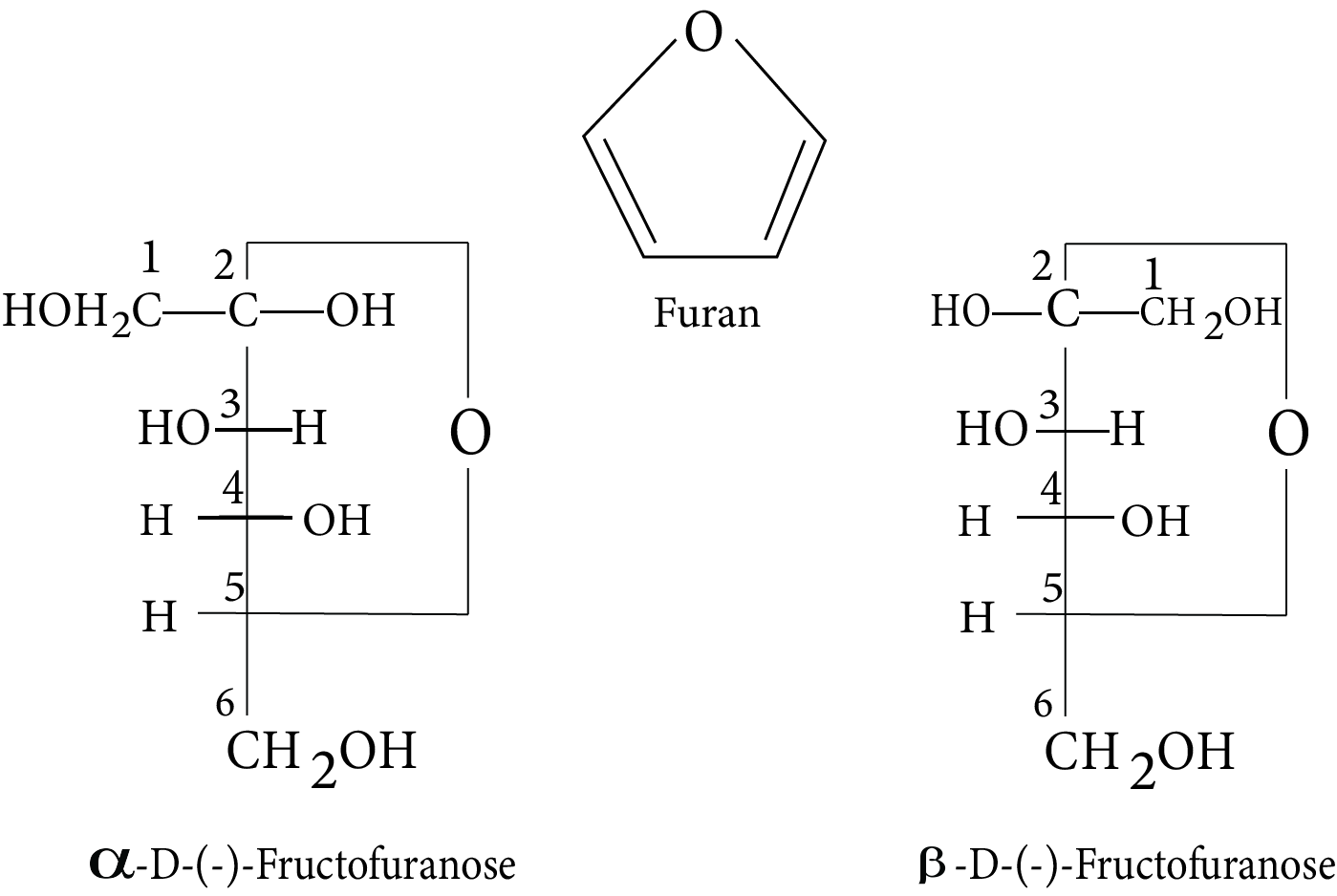
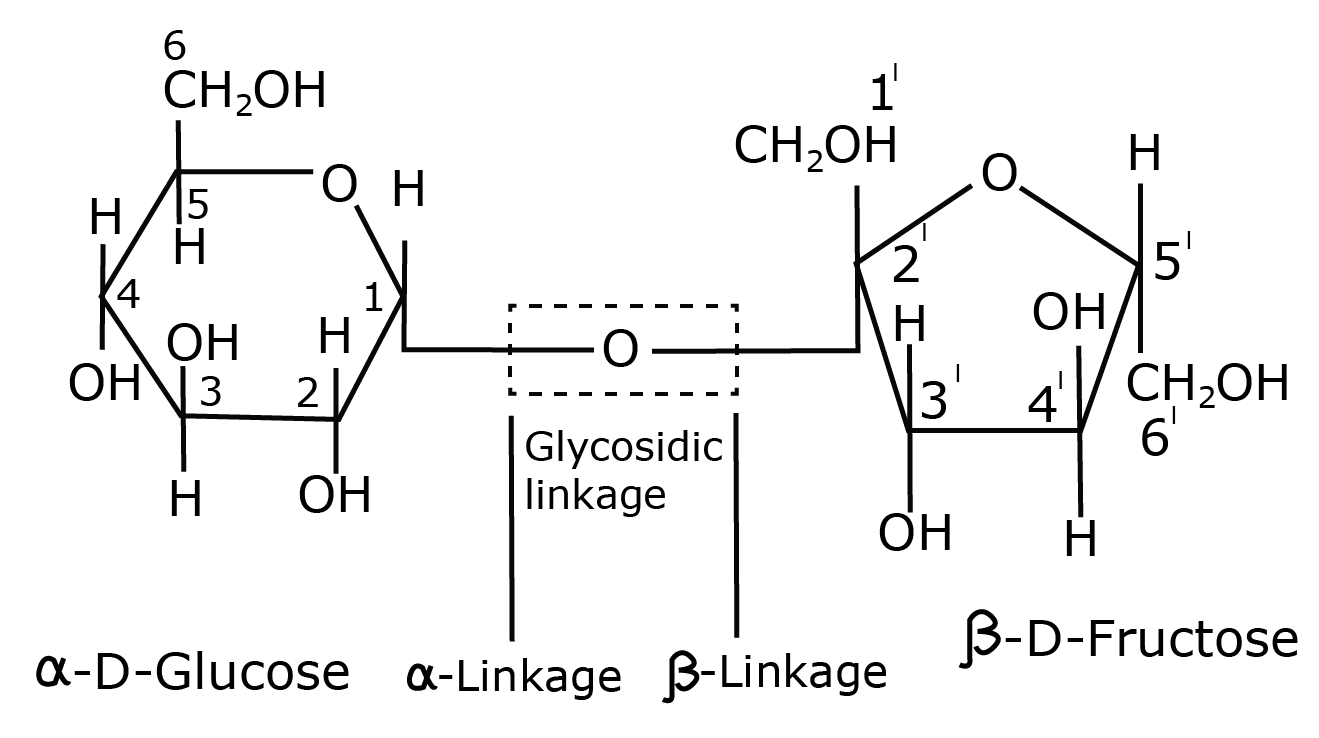
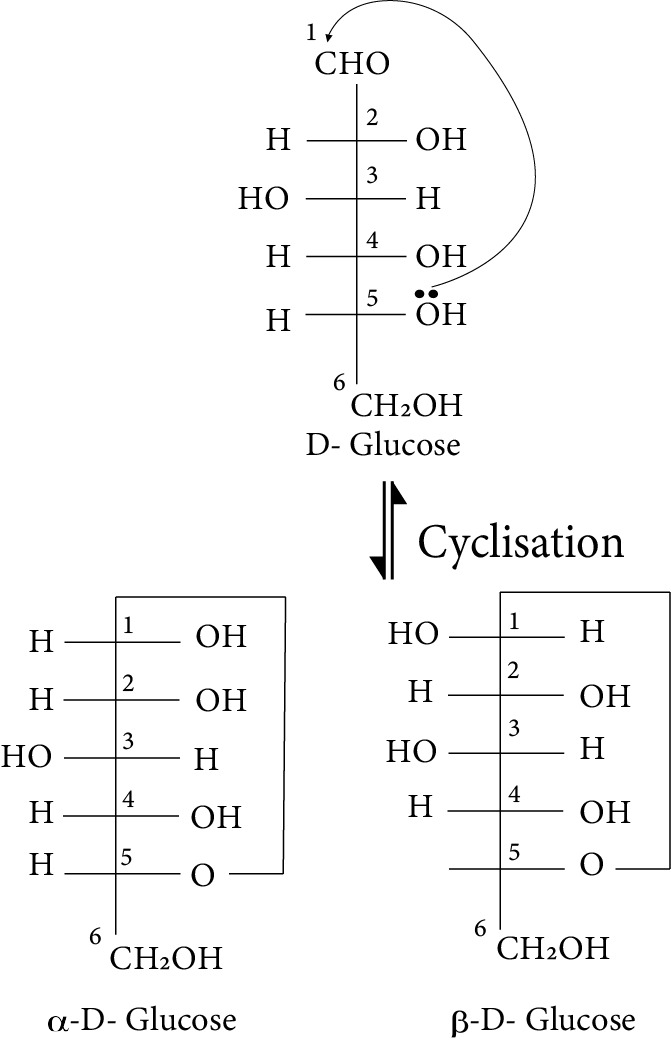
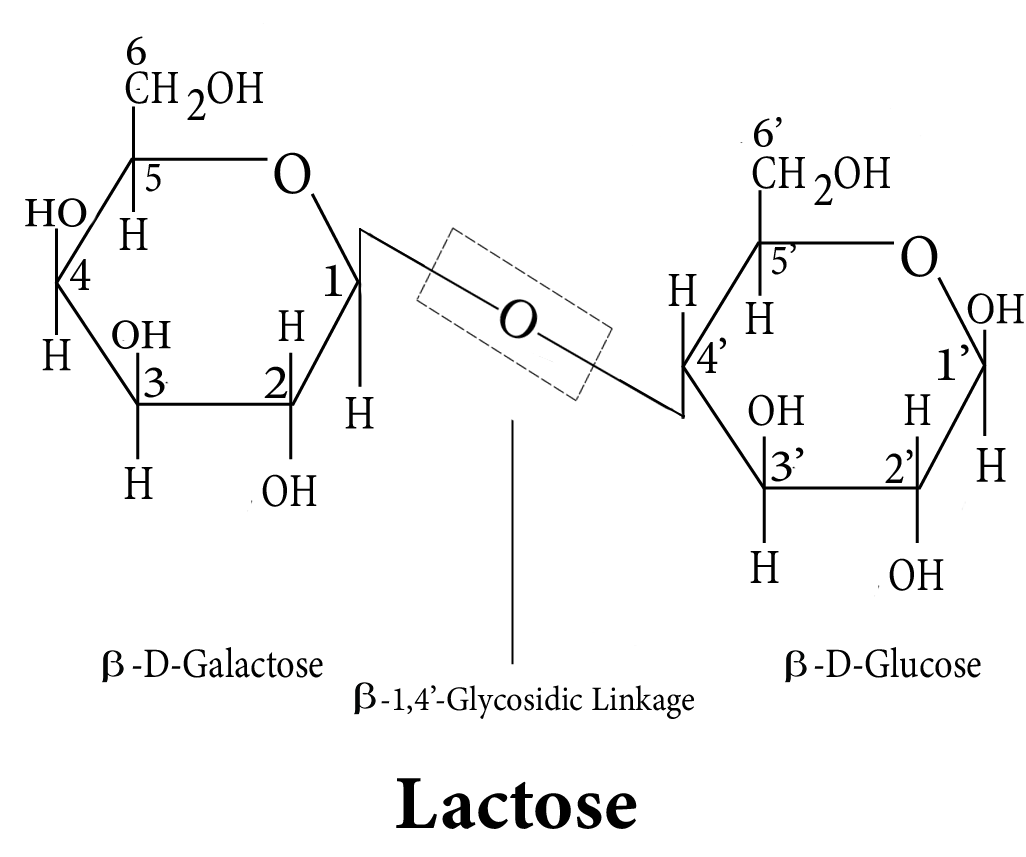
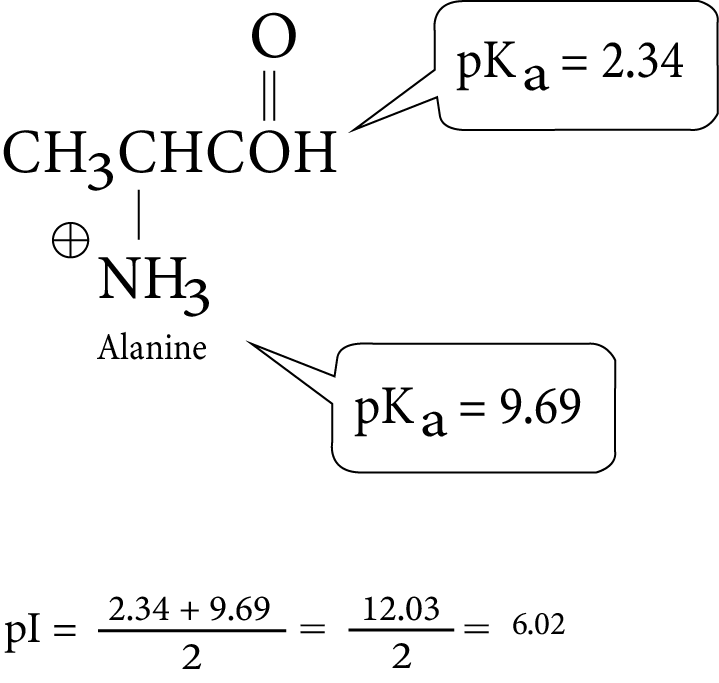
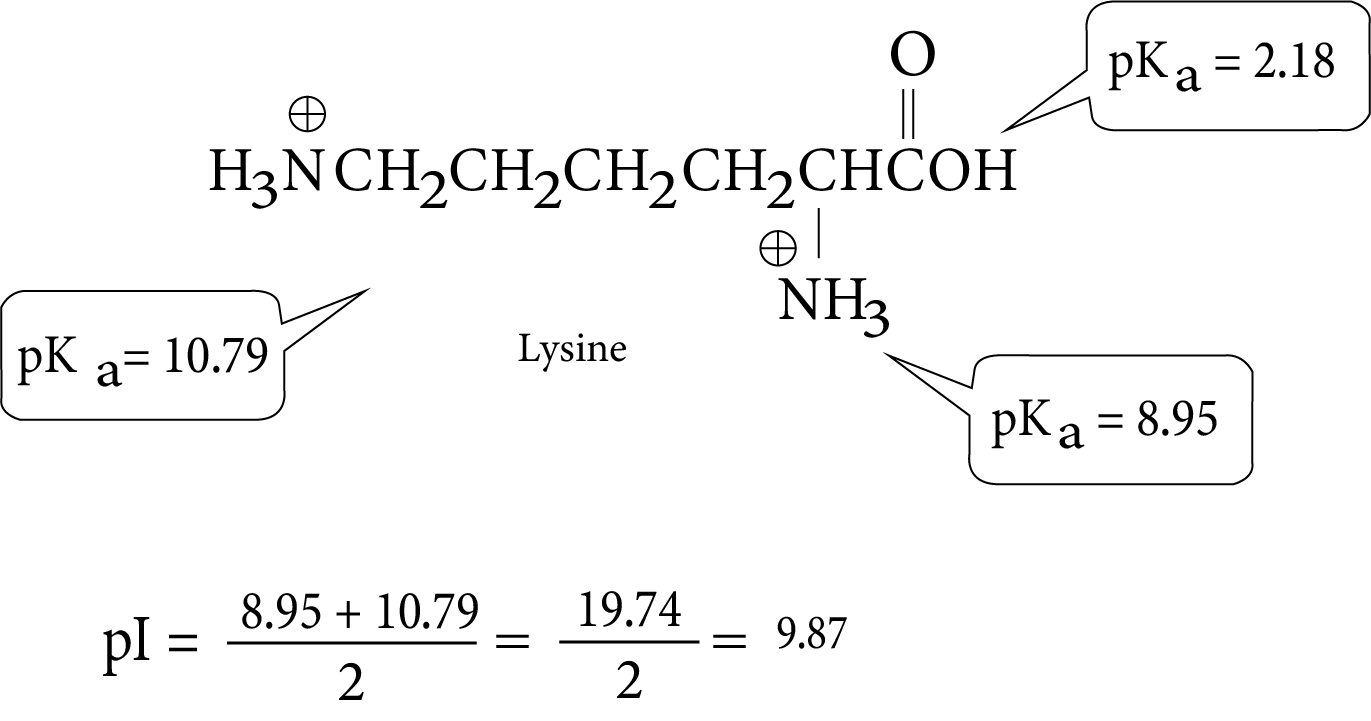
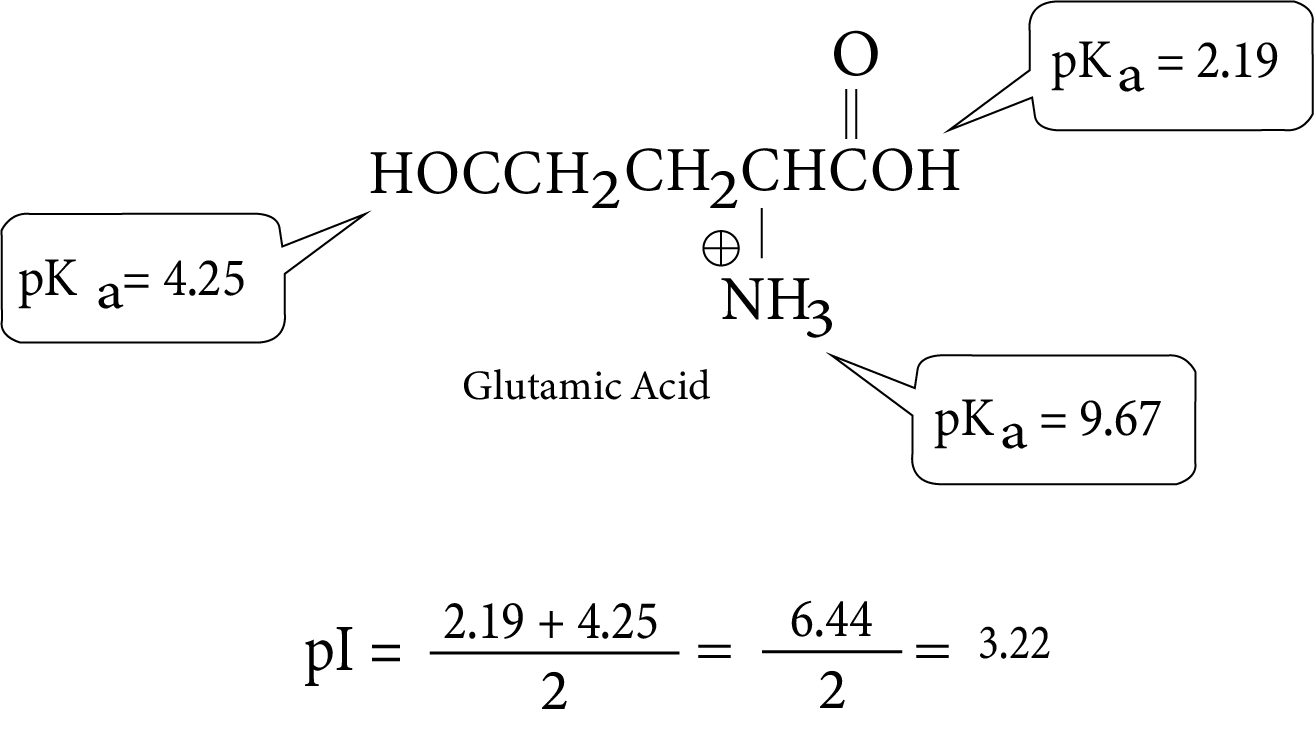
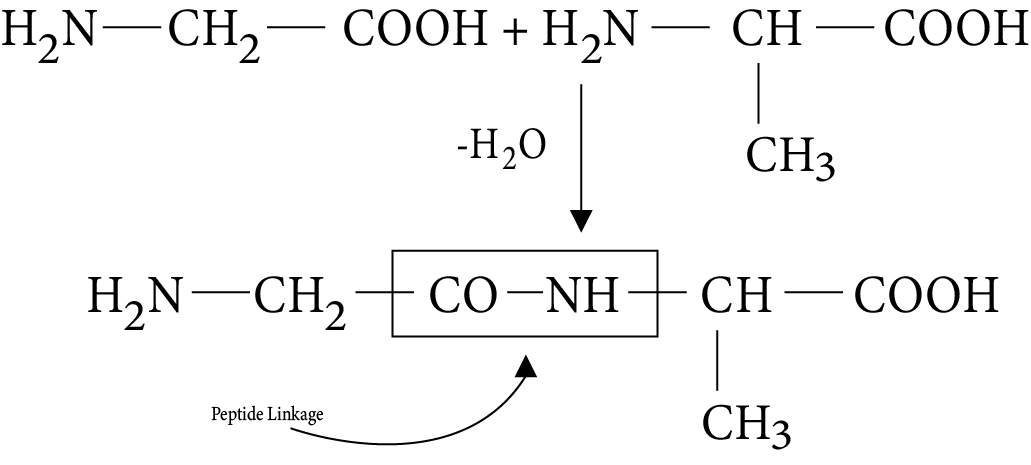
- Key definitions: monosaccharides, disaccharides, polysaccharides, peptide bond, nucleotide, mutarotation
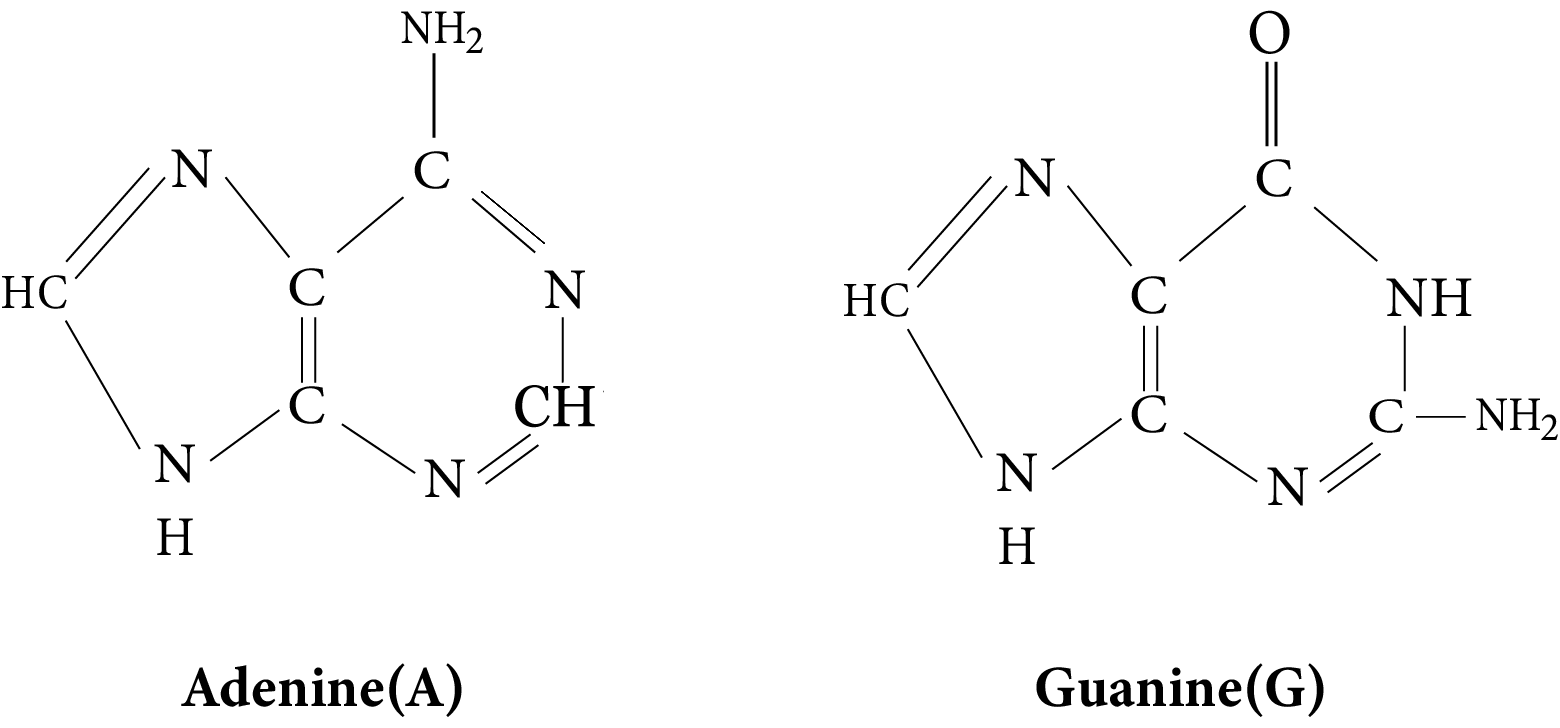
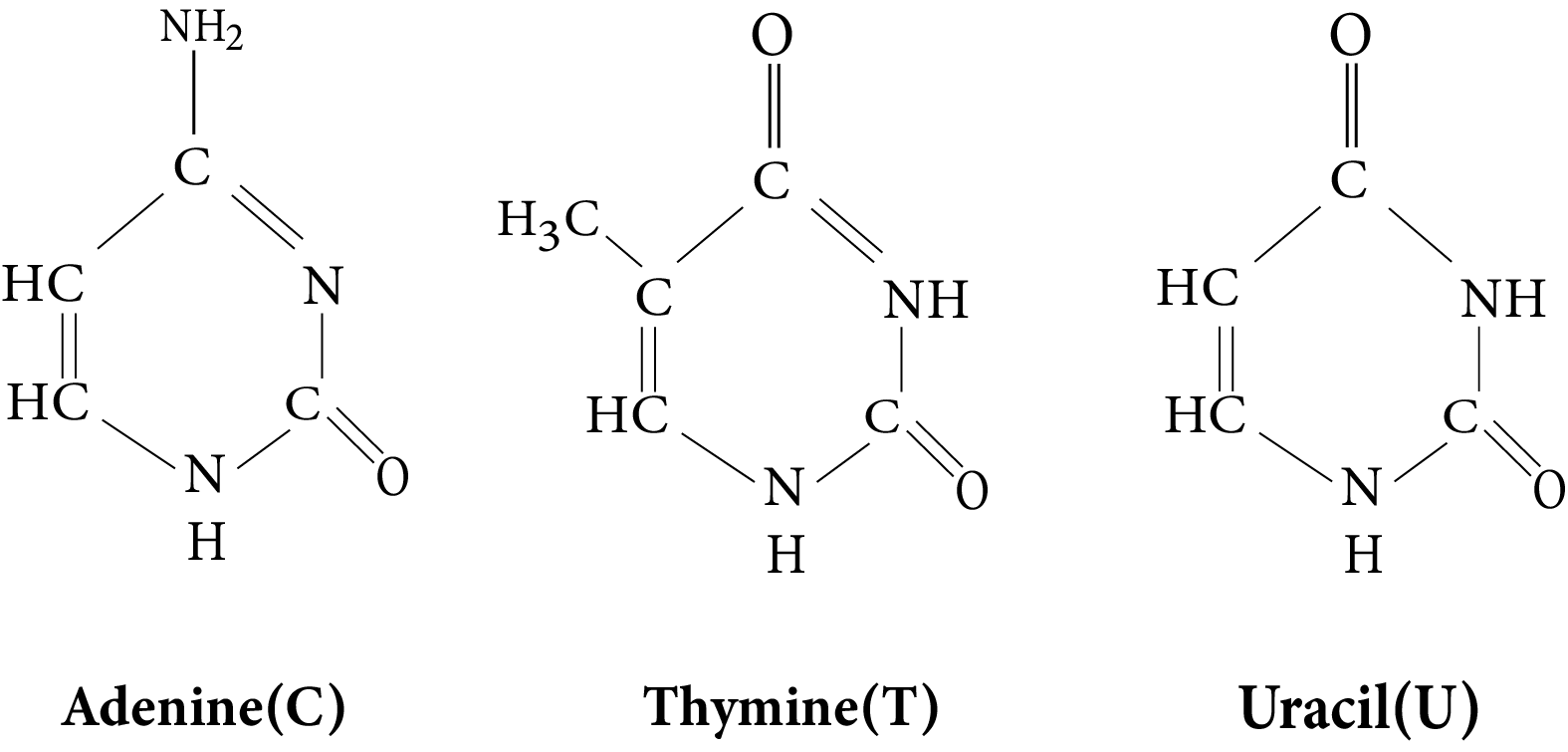
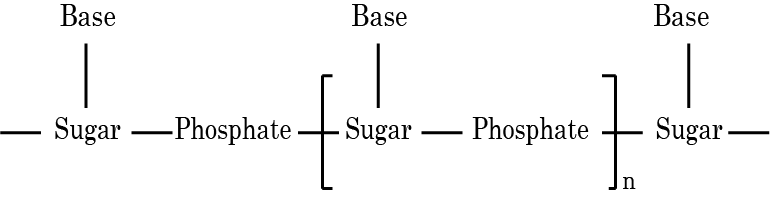
- Structures and functions of glucose, starch, cellulose, DNA, and RNA
- The four levels of protein structure (primary, secondary, tertiary, quaternary)
- Common reactions—hydrolysis, saponification, peptide bond formation
- Role and significance of biomolecules in living systems
4. How can students use concept maps to connect topics while revising Biomolecules?
Creating concept maps helps visually link related topics—for example, mapping the relationship between types of carbohydrates, their structure, and biological function. Linking protein structure levels to their roles, or connecting DNA structure with genetic information flow, makes interconnections clear. These visual tools aid memory retention, clarify how ideas are related, and allow for efficient last-minute revision.
5. What are common misconceptions students face when studying biomolecules and how can these be avoided through notes?
Students often believe all sugars are reducing, confuse DNA with RNA functions, or overlook the difference between peptide and glycosidic bonds. They might also think all proteins have similar structures. Summarized notes should clearly distinguish reducing vs non-reducing sugars, specify the roles of DNA vs RNA, and explain different bond types and protein structure levels to address these misconceptions.
6. Why is understanding the four levels of protein structure crucial during rapid revision?
Recognizing primary, secondary, tertiary, and quaternary protein structures is essential because each level determines the protein's function, stability, and ability to interact in biological processes. For example, denaturation affects secondary and tertiary structures, impacting enzyme activity. Quick recall of these distinctions supports strong answers to application-based and reasoning questions in board exams.
7. How should real-life examples be used in summarising the functions of different biomolecules?
Link each biomolecule to a specific function and real-life context: starch is an energy reserve in plants, cellulose gives structural support to plant cell walls, glycogen stores energy in animals, and insulin regulates blood sugar. Using relatable examples makes it easier to remember both structure and function during revision.
8. How do summary notes address application-based and HOTS questions in Biomolecules?
Well-organized summary notes not only cover factual content but also include conceptual connections, flowcharts, or diagrams that clarify structure–function relationships and biological significance. By including example-based questions or 'reasoning why' a process occurs, notes prepare students for higher-order thinking and application-based CBSE 2025–26 exam items.
9. What revision strategies are recommended for mastering the detailed reactions and structures within Biomolecules?
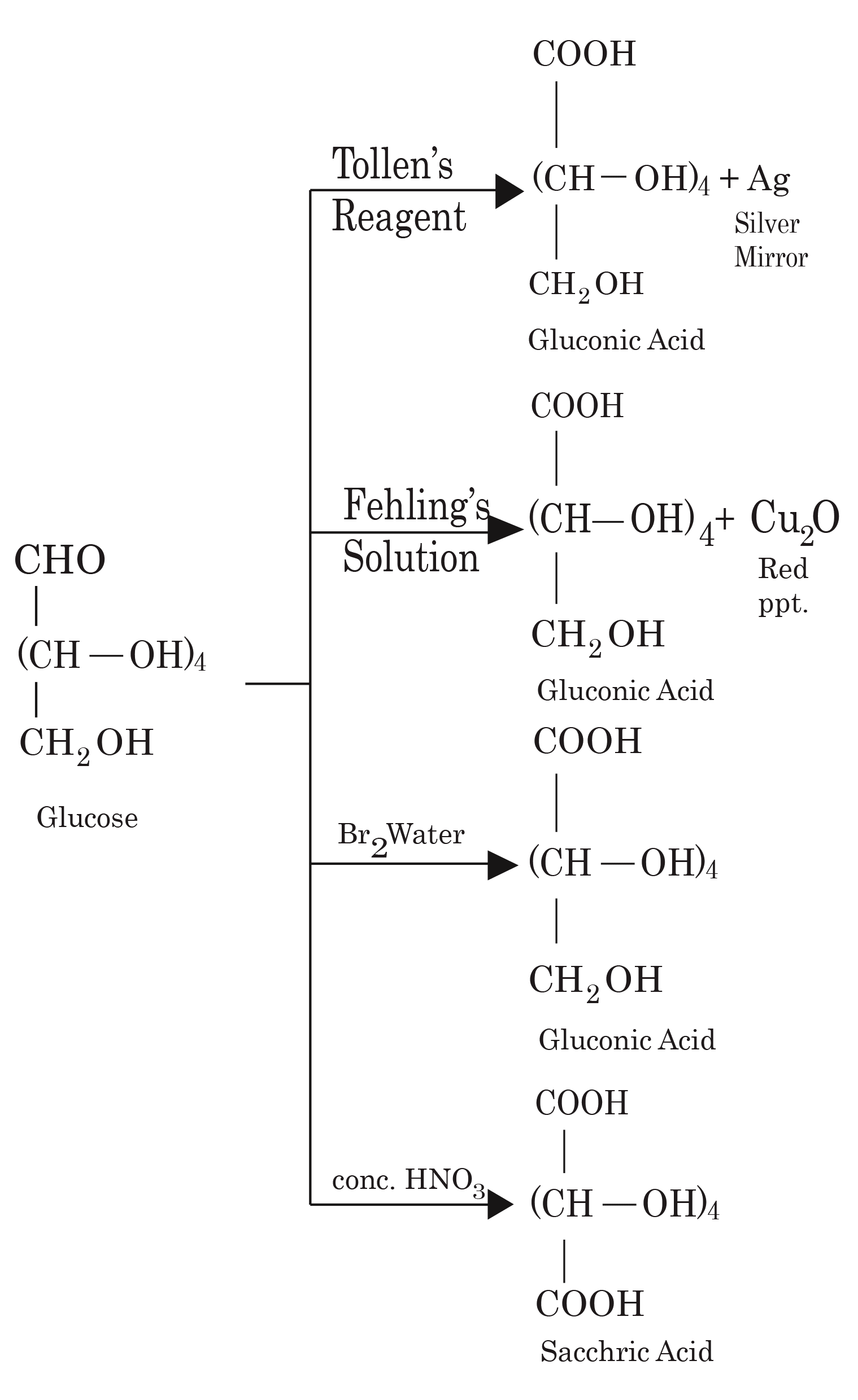
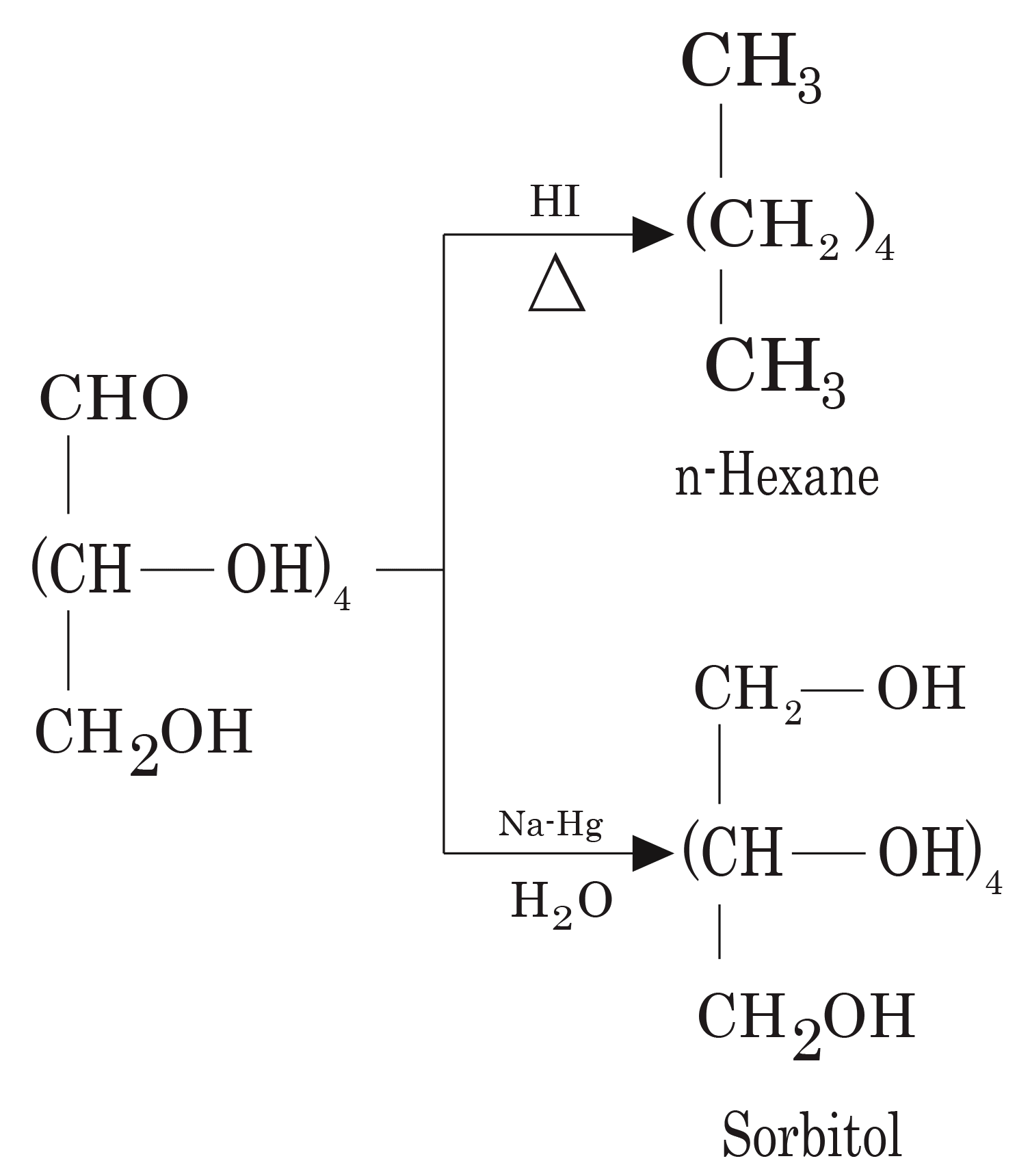
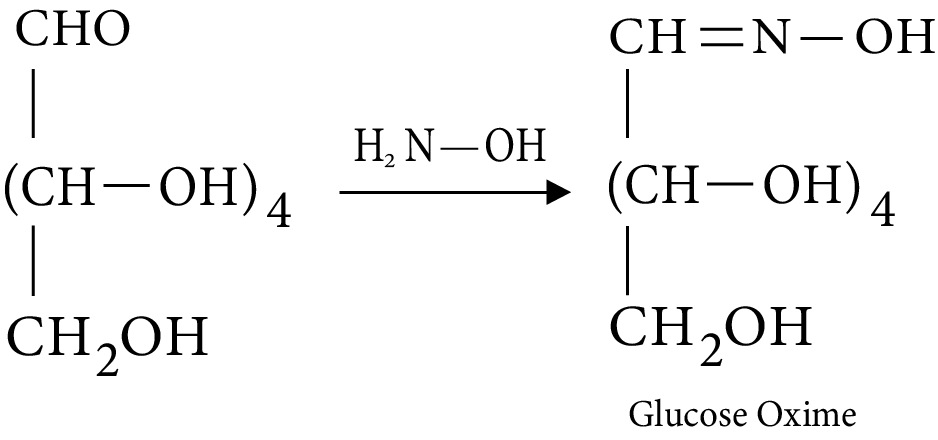
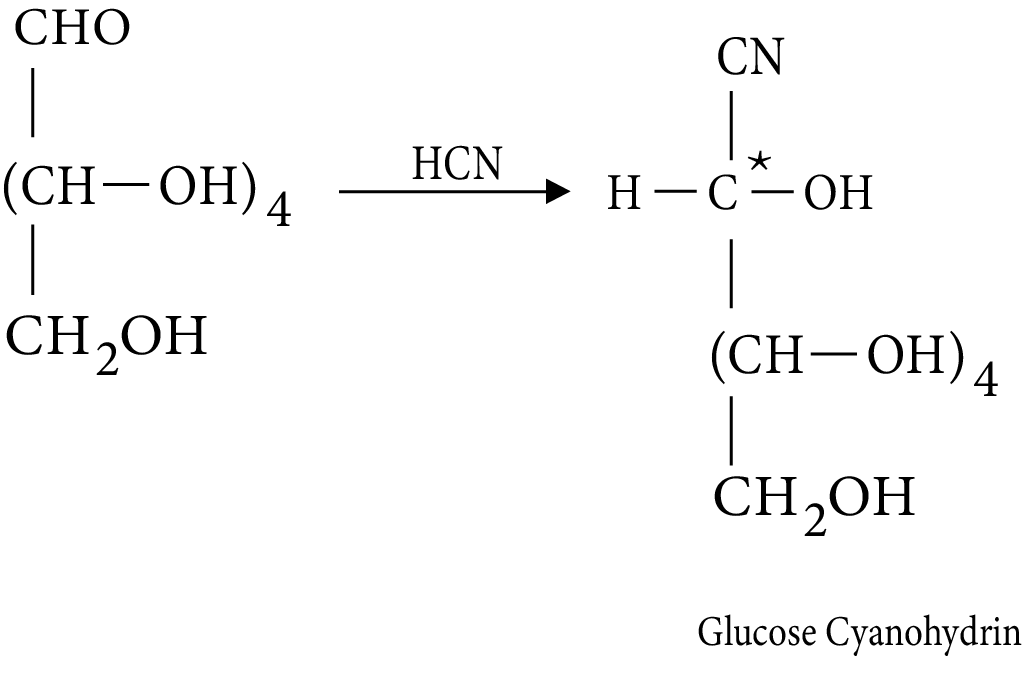
Use repetition and stepwise writing to memorize reactions (such as hydrolysis of sucrose, peptide bond formation, saponification). Draw labelled diagrams for the structures of glucose, amino acids, or DNA. Break complex reactions into simple, logical steps and revise them regularly for long-term retention. Practice explaining these reactions out loud or teaching them to someone else for deeper understanding.
10. How can students ensure their revision covers all essential Biomolecules concepts as outlined in the CBSE/NCERT 2025–26 syllabus?
Cross-check their summary notes with the latest official CBSE/NCERT Chemistry syllabus. Make sure each biomolecule type (carbohydrates, proteins, lipids, nucleic acids) is included, along with their definitions, structures, main reactions, and biological roles. Prioritize concepts mentioned in the chapter overview and highlighted in the official syllabus to ensure nothing is missed before the exam.