
Assertion: In tetrahedral complexes low spin configurations are rarely observed.
Reason: ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}=\dfrac{4}{9}\Delta \text{o}$
A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
B) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
C) Assertion is correct but Reason is incorrect
D) Both Assertion and Reason are incorrect
Answer
232.8k+ views
Hint: In tetrahedral complexes, the ligand approaches in-between of the axes of a tetrahedron. The d orbital in the direction of the ligand experiences more repulsion and the d-orbital loses its degeneracy. The energy associated with tetrahedral complexes is less than that of the octahedral complex.
Complete step by step answer:
-In the case of free metal ions, all the d-orbitals have the same energy. These orbitals having the same energies are called a degenerate orbital. However, on the approach of the ligand, the electrons get repelled by the lone pairs of the ligand. This repulsion will raise the energy of d-orbital. The orbitals lying in the direction of ligands experience greater repulsion. On the other hand, the orbitals lying away from the approach of ligand experience lesser interactions.
-The conversion of five degenerate-orbitals of the metal into different sets of orbital having different energies in the presence of an electrical field is called the crystal splitting.
-Let us consider a tetrahedral complex. In the tetrahedral complex, the metal ion is at the center of the tetrahedron and the ligands are at the corners of the tetrahedron.
-In tetrahedral complexes, none of the d-orbitals points exactly towards the ligands, and therefore, the splitting of energy will be less than that of the octahedral field. The three d-orbitals $({{d}_{xy}},{{d}_{yz}}\text{ and }{{\text{d}}_{\text{xz}}})$ are pointing close to the direction in which the ligands are approaching while the two d-orbitals $({{d}_{{{x}^{2}}-{{y}^{2}}}}\text{, }{{\text{d}}_{{{\text{z}}^{\text{2}}}}})$ are lying between the ligands. Therefore, the energies of the three orbitals will be raised while the energies of the two orbitals will be lowered. Thus, in the presence of tetrahedral field the degeneracy of d-orbital split us as:
(i) The two d-orbitals $({{d}_{{{x}^{2}}-{{y}^{2}}}}\text{ and }{{\text{d}}_{{{\text{z}}^{\text{2}}}}})$ become more stable and their energies are lowered. These are designed as ‘e’ orbitals.
(ii)The three orbitals $({{d}_{xy}},{{d}_{yz}}\text{ and }{{\text{d}}_{\text{xz}}})$ become less stable and their energies are raised. These are designated as ‘\[{{t}_{2}}\]’ orbitals. This splitting of d-orbital is shown below:
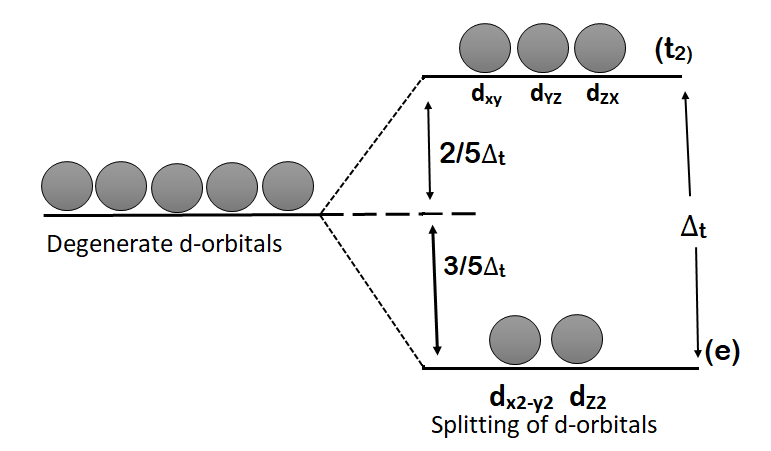
-The energy difference between these two sets is called crystal field splitting in the tetrahedral field and is abbreviated as ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}$ where subscript ‘t’ indicates tetrahedral complexes. Crystal field splitting in tetrahedral complexes ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}$ is smaller than the octahedral complexes ${{\Delta }_{O}}$ because:
1) In tetrahedral complexes, there are four ligands while there are six ligands in octahedral complexes. Therefore, lower ligands will produce less crystal field splitting.
2) In tetrahedral complexes, none of the orbitals is pointing directly toward the ligands, and therefore, splitting is less.
Splitting in tetrahedral complexes is considerably less than in octahedral complexes. It has been found that
${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}=\dfrac{4}{9}\Delta \text{o}$
-We know that ${{\Delta }_{O}}$ represents the crystal field energy and P is the pairing energy. The strong field ligand forces the electron to get paired as the ${{\Delta }_{O}}$ is more than the pairing energy. This gives the low spin complexes.
-However, according to the relation of crystal field energies of octahedral and tetrahedral complexes, the tetrahedral complexes have a smaller energy barrier between d-orbitals. The crystal field stabilization energy (CFSE) for tetrahedral complexes is less than the pairing energy. Thus electrons occupy the higher energy orbital. This is because the energy required for the pairing of electrons in the lower orbital is greater than the CFSE.The orbital splitting energy in tetrahedral complexes is not enough for spin pairing.
Thus tetrahedral complexes easily form the high spin complexes, but rarely low spin complexes.
Hence, the assertion and reason are correct.
Note: One should remember that, in tetrahedral complexes, the no of ligands surrounding metal are four. Thus the number of the ligands is $\dfrac{2}{3}rd$ times the no of ligands in octahedral complexes. Thus the energy of the tetrahedral complex is lesser than that of octahedral complexes. Since the energy is less it can easily overcome \[{{\Delta }_{o}}\] and readily forms high spin complex but rarely low spin complexes.
Complete step by step answer:
-In the case of free metal ions, all the d-orbitals have the same energy. These orbitals having the same energies are called a degenerate orbital. However, on the approach of the ligand, the electrons get repelled by the lone pairs of the ligand. This repulsion will raise the energy of d-orbital. The orbitals lying in the direction of ligands experience greater repulsion. On the other hand, the orbitals lying away from the approach of ligand experience lesser interactions.
-The conversion of five degenerate-orbitals of the metal into different sets of orbital having different energies in the presence of an electrical field is called the crystal splitting.
-Let us consider a tetrahedral complex. In the tetrahedral complex, the metal ion is at the center of the tetrahedron and the ligands are at the corners of the tetrahedron.
-In tetrahedral complexes, none of the d-orbitals points exactly towards the ligands, and therefore, the splitting of energy will be less than that of the octahedral field. The three d-orbitals $({{d}_{xy}},{{d}_{yz}}\text{ and }{{\text{d}}_{\text{xz}}})$ are pointing close to the direction in which the ligands are approaching while the two d-orbitals $({{d}_{{{x}^{2}}-{{y}^{2}}}}\text{, }{{\text{d}}_{{{\text{z}}^{\text{2}}}}})$ are lying between the ligands. Therefore, the energies of the three orbitals will be raised while the energies of the two orbitals will be lowered. Thus, in the presence of tetrahedral field the degeneracy of d-orbital split us as:
(i) The two d-orbitals $({{d}_{{{x}^{2}}-{{y}^{2}}}}\text{ and }{{\text{d}}_{{{\text{z}}^{\text{2}}}}})$ become more stable and their energies are lowered. These are designed as ‘e’ orbitals.
(ii)The three orbitals $({{d}_{xy}},{{d}_{yz}}\text{ and }{{\text{d}}_{\text{xz}}})$ become less stable and their energies are raised. These are designated as ‘\[{{t}_{2}}\]’ orbitals. This splitting of d-orbital is shown below:

-The energy difference between these two sets is called crystal field splitting in the tetrahedral field and is abbreviated as ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}$ where subscript ‘t’ indicates tetrahedral complexes. Crystal field splitting in tetrahedral complexes ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}$ is smaller than the octahedral complexes ${{\Delta }_{O}}$ because:
1) In tetrahedral complexes, there are four ligands while there are six ligands in octahedral complexes. Therefore, lower ligands will produce less crystal field splitting.
2) In tetrahedral complexes, none of the orbitals is pointing directly toward the ligands, and therefore, splitting is less.
Splitting in tetrahedral complexes is considerably less than in octahedral complexes. It has been found that
${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{t}}}=\dfrac{4}{9}\Delta \text{o}$
-We know that ${{\Delta }_{O}}$ represents the crystal field energy and P is the pairing energy. The strong field ligand forces the electron to get paired as the ${{\Delta }_{O}}$ is more than the pairing energy. This gives the low spin complexes.
-However, according to the relation of crystal field energies of octahedral and tetrahedral complexes, the tetrahedral complexes have a smaller energy barrier between d-orbitals. The crystal field stabilization energy (CFSE) for tetrahedral complexes is less than the pairing energy. Thus electrons occupy the higher energy orbital. This is because the energy required for the pairing of electrons in the lower orbital is greater than the CFSE.The orbital splitting energy in tetrahedral complexes is not enough for spin pairing.
Thus tetrahedral complexes easily form the high spin complexes, but rarely low spin complexes.
Hence, the assertion and reason are correct.
Note: One should remember that, in tetrahedral complexes, the no of ligands surrounding metal are four. Thus the number of the ligands is $\dfrac{2}{3}rd$ times the no of ligands in octahedral complexes. Thus the energy of the tetrahedral complex is lesser than that of octahedral complexes. Since the energy is less it can easily overcome \[{{\Delta }_{o}}\] and readily forms high spin complex but rarely low spin complexes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




