
Assertion: Zinc blende and wurtzite both have fcc arrangement of \[{{\text{S}}^{2 - }}\] ions.
Reason: A unit cell of both has four formula units ${\text{ZnS}}$
(A) Both assertion and reason are correct and reason is the correct explanation of assertion
(B) Both assertion and reason are correct but reason is not the correct explanation of assertion.
(C) Assertion is correct but reason is not correct.
(D) Assertion is not correct but reason is correct.
(E) Both assertion and reason are not correct.
Answer
233.1k+ views
Hint: Zinc blend has the formula ${\text{ZnS}}$. In a zinc blend structure, ${\text{Z}}{{\text{n}}^{{\text{2 + }}}}$ and ${{\text{S}}^{2 - }}$ ions are arranged in a crystal lattice. Wurtzite has the formula ${\text{(Zn,Fe)S}}$.
Complete step by step answer:
A closely packed cubic arrangement in which the facial positions are equivalent to each of the eight corners is known as a face centred cubic arrangement.
A closely packed arrangement in two dimensions in which each sphere has six nearest neighbours and has a hexagonal symmetry is known as a hexagonal close packing arrangement.
Zinc blend has a face centred cubic (fcc) arrangement of ${{\text{S}}^{2 - }}$ ions. Wurtzite has hexagonal close packing (hcp) arrangement of ${{\text{S}}^{2 - }}$ ions.
The smallest repeating unit of the structure in a crystal lattice is known as unit cell.
The unit cell of zinc blend has four formula units of ${\text{ZnS}}$. The unit cell of wurtzite has six formula units of ${\text{ZnS}}$.
The structures of zinc blende and wurtzite are as follows:
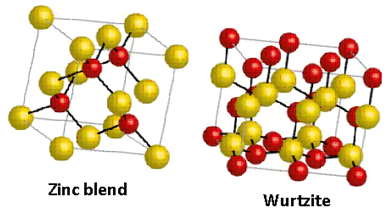
Thus, the assertion and reason both are not correct.
Thus, the correct option is (E) both assertion and reason are not correct.
Additional Information:
There are three types of unit cells:
1. Primitive cubic unit cell: The atoms are present at the eight corners of a cube.
2. Body centred cubic unit cell: The atoms are present at the eight corners of a cube and one atom is present at the centre of the structure.
3. Face centred cubic unit cell: The atoms are present at the eight corners of a cube and at the centre of all the six faces of a cube.
Note: In hcp, there are two layers of ions or atoms while in fcc there are three layers. The hcp structure has two planes while the fcc structure has three planes. The coordination number of hcp structure is $12$ and that of fcc structure is also $12$.
Complete step by step answer:
A closely packed cubic arrangement in which the facial positions are equivalent to each of the eight corners is known as a face centred cubic arrangement.
A closely packed arrangement in two dimensions in which each sphere has six nearest neighbours and has a hexagonal symmetry is known as a hexagonal close packing arrangement.
Zinc blend has a face centred cubic (fcc) arrangement of ${{\text{S}}^{2 - }}$ ions. Wurtzite has hexagonal close packing (hcp) arrangement of ${{\text{S}}^{2 - }}$ ions.
The smallest repeating unit of the structure in a crystal lattice is known as unit cell.
The unit cell of zinc blend has four formula units of ${\text{ZnS}}$. The unit cell of wurtzite has six formula units of ${\text{ZnS}}$.
The structures of zinc blende and wurtzite are as follows:

Thus, the assertion and reason both are not correct.
Thus, the correct option is (E) both assertion and reason are not correct.
Additional Information:
There are three types of unit cells:
1. Primitive cubic unit cell: The atoms are present at the eight corners of a cube.
2. Body centred cubic unit cell: The atoms are present at the eight corners of a cube and one atom is present at the centre of the structure.
3. Face centred cubic unit cell: The atoms are present at the eight corners of a cube and at the centre of all the six faces of a cube.
Note: In hcp, there are two layers of ions or atoms while in fcc there are three layers. The hcp structure has two planes while the fcc structure has three planes. The coordination number of hcp structure is $12$ and that of fcc structure is also $12$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




