




Introduction to an Atom
What is an atom? John Dalton in 1808 considered that all matter was composed of small particles called atoms. He visualised the atom as a hard and solid individual particle that is incapable of subdivision. At the end of the 19th century, he accumulated enough experimental evidence to show that the atom is made of still smaller particles. These subatomic particles are called the fundamental particles of the atom. Therefore, the three most important subatomic particles are the proton, neutron, and electron.
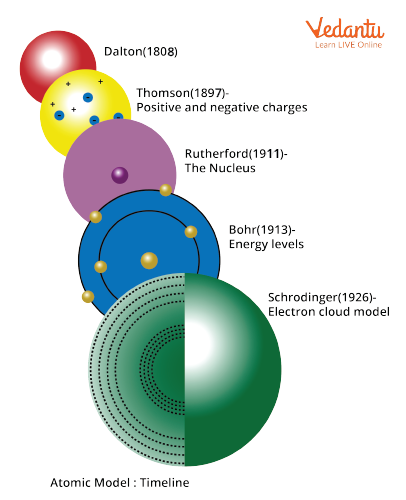
Timeline of Discovery of Atom
Mass of an Electron
By using the Thomson’s value of $\dfrac{e}{m}$ and the Milikan’s value of e, the absolute mass of an electron can be found.
$\dfrac{e}{m}$ = – 1.76 $\times$ 108 $\dfrac{coulomb}{g}$ (Thomson)
e = – 1.60 $\times$ 10-19 coulomb (Milikan)
$\therefore \dfrac{e}{\dfrac{e}{ m}}=\dfrac{1.60 \times 10^{-19}}{1.76 \times 10^{8}}$
Hence, m = 9.1 $\times$ 10-28 g or 9.1 $\times$ 10-31 kg
Definition of an Electron
By knowing the charge and mass of an electron, the electron can be defined as:
An electron is a subatomic particle that bears a charge of – 1.60 $\times$ 10-19 coulomb and has a mass of 9.1 $\times$ 10-28 g.
In other words, an electron can be defined as:
A particle that bears a unit negative charge and mass that is $\dfrac{1}{1835^{th}}$ of a hydrogen atom. Since an electron has the smallest charge that is known ever, it was designated as a unit charge by Thomson.
Protons
E. Goldstein in the year 1886 discovered protons in the discharge tube containing hydrogen atoms.
$\mathrm{H} \rightarrow \underset{\text{Proton}}{\mathrm{H}^{+}}+e^{-}$
It was J.J. Thomson who studied their nature. He showed that :
The actual mass of a proton is 1.672 $\times$ 10-24 gram. On the relative scale, protons have mass 1 atomic mass unit (amu).
The electrical charge of a proton is equal in magnitude but opposite to that of the electron.
Thus, the proton carries a charge +1.60 $\times$ 10-19 coulombs or +1 elementary charge unit. Since the proton was the lightest positive particle found in atomic beams in the discharge tube, it was thought to be a unit present in all other atoms. By a variety of nuclear reactions indicating further that all atoms contain protons, they can be obtained. Thus, a proton can be defined as a subatomic particle which has a mass of 1 atomic mass unit and charge of +1 elementary charge unit. A proton is evidently a subatomic particle which has one unit mass and one unit positive charge.
Neutrons
In the year 1932, Sir James Chadwick discovered another third subatomic particle. He directed a stream of alpha particles (2He4)2+ at a beryllium target. A new particle that was ejected was found by him. It has almost the same mass that is 1.674 $\times$ 10-24 g as that of a proton and has no charge. He named it neutron.
The relative mass of a neutron assigned is approximately one atomic mass unit (amu). Thus, a neutron is also a subatomic particle, which has a mass that is almost equal to that of a proton and has no charge. The reaction which occurred in the experiment performed by Chadwik is an example of artificial transmutation, where an atom of beryllium is converted to a carbon atom through the following nuclear reaction.
${ }_{2}^{4} \mathrm{He}+{ }_{4}^{9} \mathrm{Be} \rightarrow{ }_{6}^{12} \mathrm{C}+{ }_{0}^{1} \mathrm{n}$
Subatomic Particles
Now, we have studied the properties of the three principal fundamental particles of the atom, namely the electron, proton, and neutron. In terms of atoms consisting of electrons, protons, and neutrons, nearly all of the ordinary chemical properties of matter can be examined. Therefore, for our discussion, we will assume that an atom contains only these three principal subatomic particles.
Other Subatomic Particles
Besides electrons, protons, and neutrons, there are many other subatomic particles such as mesons, positrons, neutrinos, and antiprotons that have been discovered by scientists. A great deal of recent research in this Nuclear Chemistry field is producing a long list of still other subatomic particles with names of quarks, pions, and gluons. With each discovery, the picture of the atomic structure becomes more and more complex. Fortunately, the three particle (electron, proton, neutron) picture of the atomic model still meets the needs of most of the chemists.
Atomic and Mass Number
Atomic Masses
How much does an individual atom weigh? As atoms are too small with a diameter of 10-10 m and weigh approximately 10-27 kg, it is not possible to measure their mass directly. It has a relative scale based on a standard atom. The carbon 12 atom is considered as standard by the IUPAC (International Union of Pure and Applied Chemistry), and its mass is fixed as 12 atomic mass unit (or) u.
The unified atomic mass unit or a.m.u. is defined as one twelfth of the mass of an atom of carbon 12 in its ground state. i.e. 1 amu (or) 1u ≈ 1.6605 × 10-27 kg. In this scale, the relative atomic mass can be defined as the ratio of the average atomic mass to the unified atomic mass unit.
$\text { Relative Atomic Mass }\left(A_{r}\right)=\dfrac{\text { Average mass of an Atom }}{\text { Unified Atomic Mass }}$
Molecular Mass
Similar to the relative atomic mass of atoms, the relative molecular mass is defined as the ratio of the mass of a molecule to the unified atomic mass unit (a.m.u.). The relative molecular mass of any compound can be easily calculated, by adding the relative atomic masses of its constituent atoms.
How to Find the Mass of An Atom?
For example:
i) Relative molecular mass of hydrogen:
= 2 $\times$ (relative atomic mass of hydrogen (H2)atom)
= 2 $\times$ 1.008 u
= 2.016 u.
ii) Relative molecular mass of glucose:
(C6H12O6)
= (6$\times$12) + ( 12$\times$1.008) + (6$\times$16)
= 72+12.096+96
= 180.096 u
Conclusion
The basic unit that makes up all matter is an atom. The word ‘atom’ has been derived from the Greek word ‘a-tomio’ which means indivisible. Atoms were considered as non divisible until the discovery of subatomic particles such as electrons, protons, and neutrons.
In the situations where it is unnecessary to differentiate between protons and neutrons, these elementary particles are collectively referred to as nucleons. Thus, the mass number of an atom is equal to the total number of nucleons in the nucleus of an atom. Obviously, the mass number of an atom is generally a whole number. Since electrons have practically no mass, the entire atomic mass is due to protons and neutrons, each of which has a mass in almost exactly one unit. Therefore, the mass number of an atom can be obtained by rounding off the experimental value of atomic mass (or atomic weight) to the nearest whole number.
FAQs on Atom and Atomic Mass - JEE Important Topic
1. Describe the empirical formula and molecular formula of a compound.
The mass percentage of atoms present in the compound is given by the elemental analysis of a compound. By using the mass percentage of the compound, we can determine the empirical formula of the compound. The molecular formula of the compound can be derived from the empirical formula, by applying the molar mass of the compound.
The empirical formula of a compound is the formula that can be written with the simplest ratio of the number of different atoms, present in one molecule of any of the compounds, as subscript to the atomic symbol of the compound. The molecular formula of a compound can be described as the formula written with the actual number of different atoms, present in one molecule, as a subscript to the atomic symbol of the element or compound.
2. Explain the gram equivalent concept of atom.
The equivalent weight of an element is its molar mass divided by its combining power (valency). Gram equivalent mass of hydrogen (1.008 g mol-1l / 1 eq mol-1) is 1.008 g eq-1, oxygen (16 g mol-1/2 eq mol-1) is 8 g eq-1, or chlorine (35.5 g mol-1/1 eq mol-1) is 35.5 g eq-1. The gram equivalent mass has the unit which is g eq-1. For oxidising or reducing agents, the equivalent weight is the molar mass divided by the number of electrons gained or lost by each molecule, respectively.
The expression is used to calculate gram equivalent mass as formulated below.
$\text { Gram equivalent mass }=\dfrac{\text { Molar mass }\left(\mathrm{g} ~\mathrm{mol}^{-1}\right)}{\text { Equivalence factor }(\mathrm{eq}~\mathrm{mol}^{-1})}$
























