




Define Chemical Bonding
As we all know, molecules are made up of atoms. These atoms can either be the same atoms, making it homoatomic, or different atoms, making it heteroatomic. These atoms are held together in a molecule. Therefore, the attractive force that holds the constituents of a chemical species such as atoms or ions together, thereby making them stable, is known as a chemical bond.
Chemical bonding results in the formation of a chemical compound that is stable. The stronger the chemical bonding within a chemical compound is, the more stable the chemical compound is.
Cause of Chemical Bonding
Noble gases are known for their chemical inert nature and stability. They have 8 electrons in their outermost shell (exception: helium is a noble gas having only 2 electrons in its outermost shell). The electronic arrangement of these 8 electrons is in such a way that they are highly stable and hence do not participate in any chemical reactions.
So, for any atom to be considered stable, it has to attain the noble gas configuration. That is, they must have 8 electrons in their outermost shell and 2 electrons in their innermost shell. This can be done by the formation of chemical bonds. The atoms combine with other atoms, resulting in the formation of chemical bonds, which facilitates them in attaining the stable noble gas electronic configuration, having 8 electrons in their outermost shell. Hence, this is the main cause of chemical bonding.
The formation of a chemical bond involves only the electrons present in the outermost shell of an atom. The atom can either lose or gain electrons from another atom (via ionic or electrovalent bond formation) or mutually share electrons with another atom (via covalent bond formation) in order to attain a stable inert electronic configuration.
Types of Chemical Bond
The types of chemical bonds formed between the atoms vary based on their strengths and properties. There are basically 4 major types:
1. Electrovalent or Ionic Bond
The bond formed between two atoms by the complete transfer of electrons is defined as an electrovalent bond. When such a transfer of electrons occurs, the atom which loses the electron gains a positive charge and the atom which gains the electron has a negative charge. These atoms are now called cations and anions, respectively. Thus, the ionic bond is formed as a result of electrostatic attraction between the cation and anion.
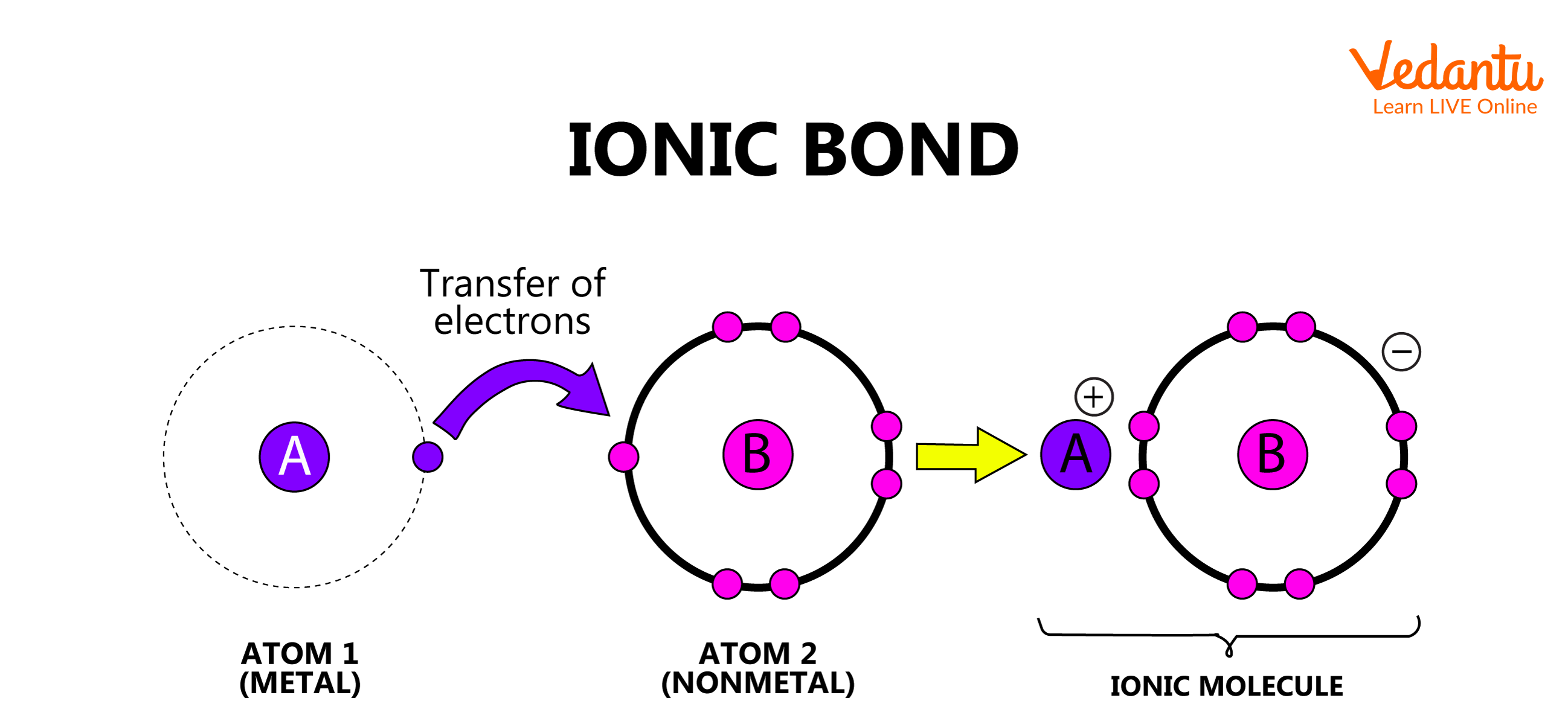
Ionic Bond
2. Covalent Bond
The bond formed between the two atoms by the mutual sharing of electrons in order to attain a noble gas configuration is defined as a covalent bond, and the number of electrons shared between them is referred to as covalency. Depending on the number of electrons shared, a single, double, or triple bond is formed between the atoms.
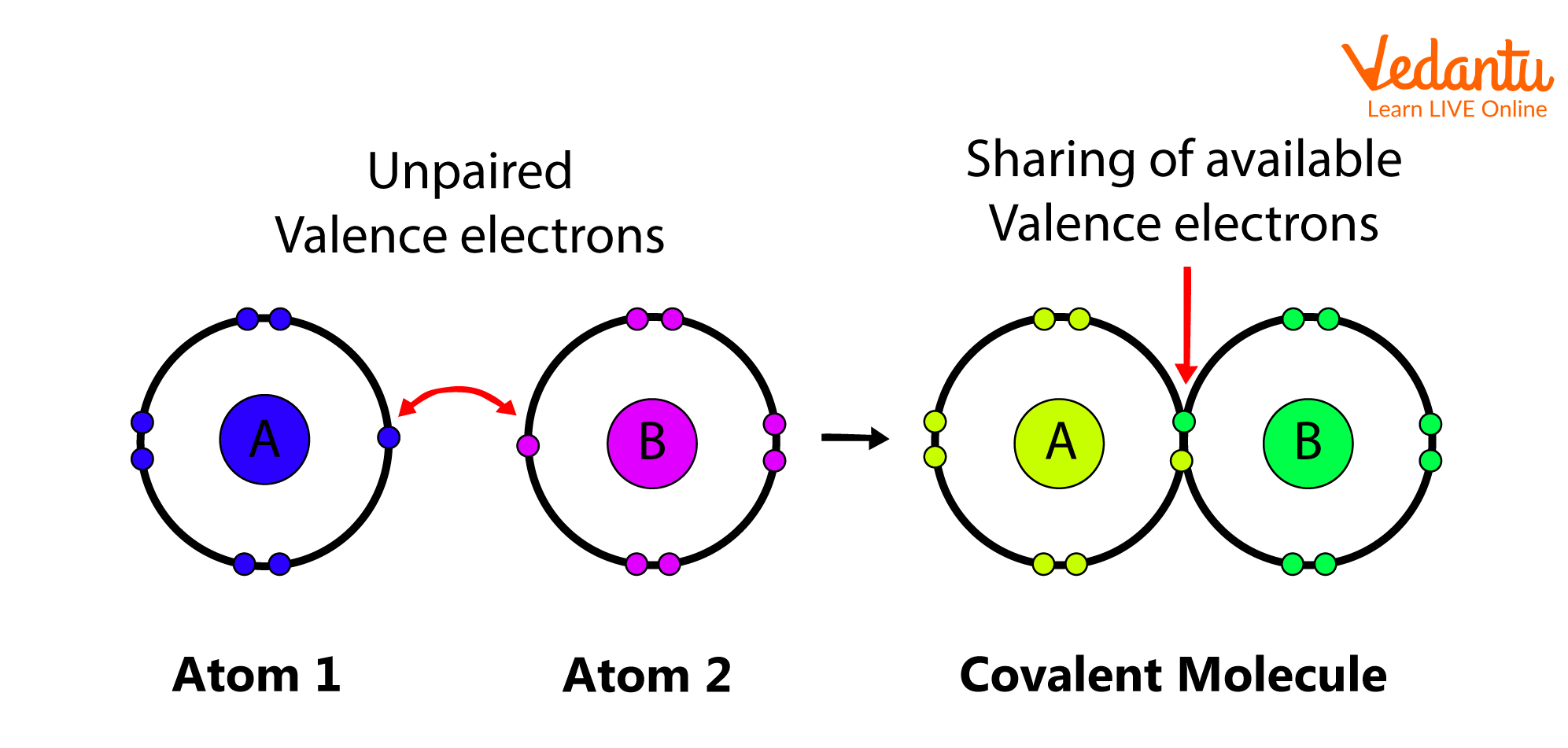
Covalent Bond
3. Hydrogen Bond
When a hydrogen atom is linked to an electronegative atom such as oxygen, nitrogen, or sulphur within a molecule, the electronegative atom tends to attract the electrons toward itself and develops a slight negative charge, whereas the hydrogen end develops a slight positive charge. Due to a weak attraction between the positive and the negative end, a weak bond is formed between them, and this bond is the hydrogen bond.
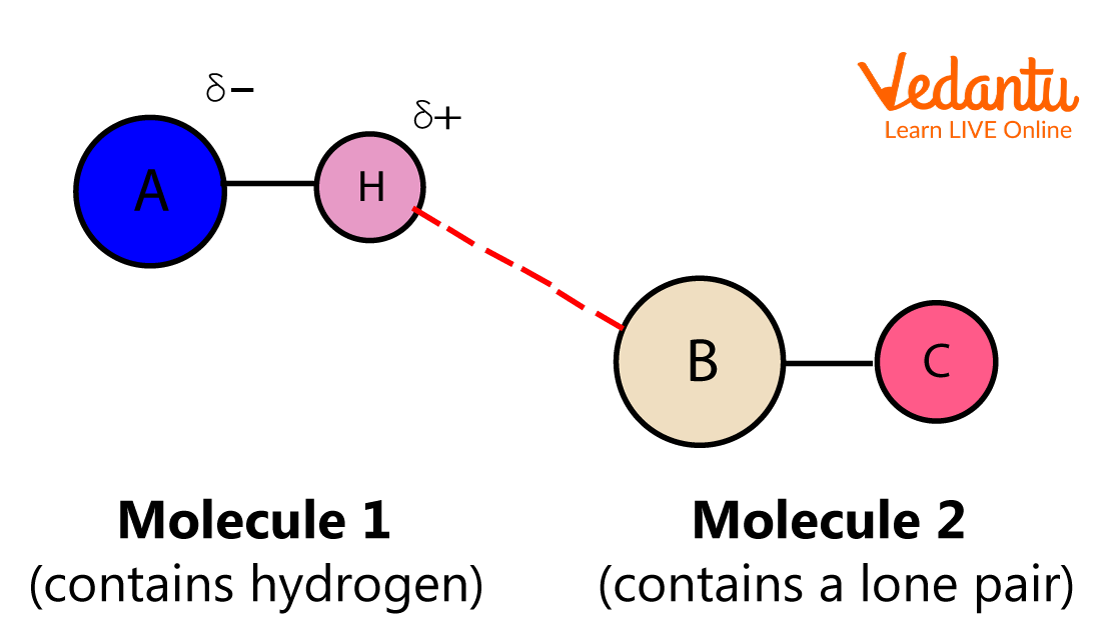
Hydrogen Bond
4. Vander Waals Interaction
Van der Waals interactions are weak interactions between molecules, similar to hydrogen bonds. These kinds of interactions occur between different molecules constituting polar, covalently linked atoms. As the distance between the atoms increases, the Van der Waals interaction disappears.
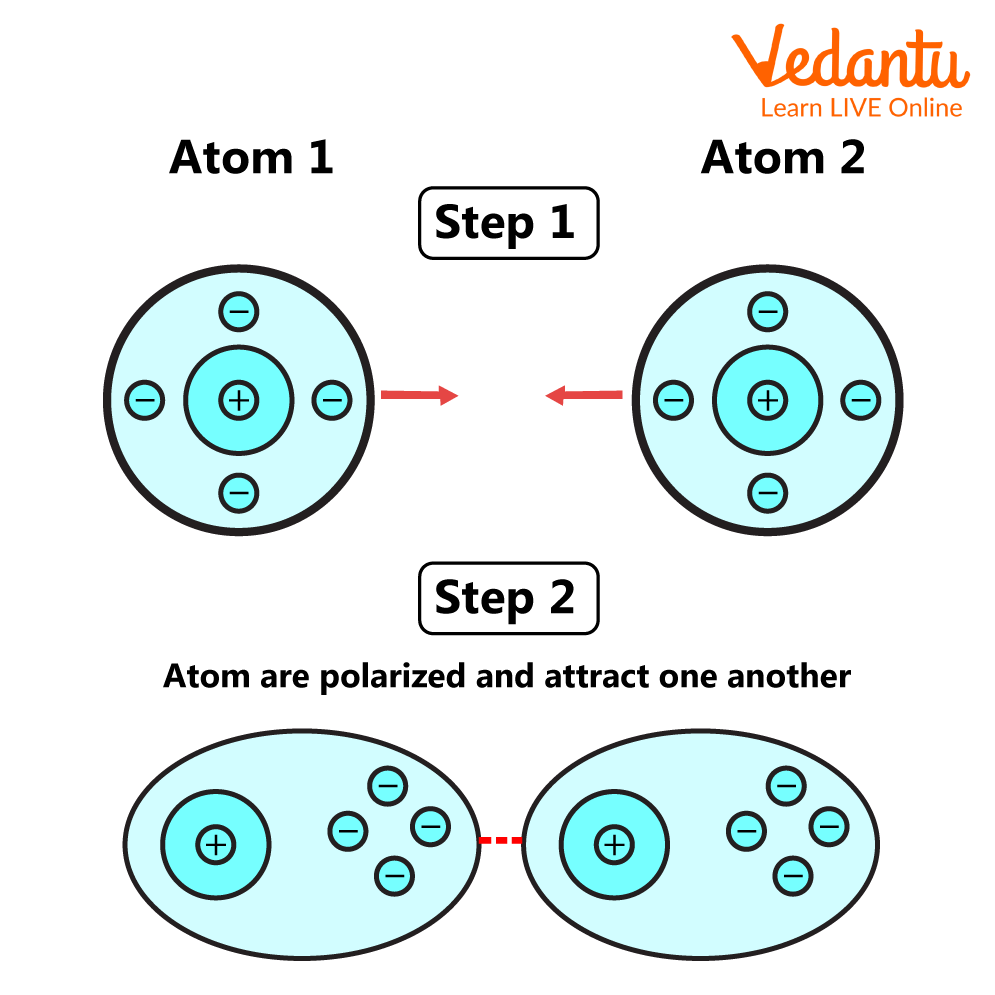
Van Der Waals Interaction
Chemical Combination and Cause of Chemical Combination
A chemical combination is the interaction or reaction between two or more elements that combine together to form a chemical compound or product. For the combination to occur, the mass of one element reacts with the constant mass of another element in a simple ratio.
Example: Sulphur reacts with oxygen to form a sulphur dioxide compound.
$\mathrm{S}+\mathrm{O}_{2} \rightarrow \mathrm{SO}_{2}$
Now we'll look at the causes of chemical combinations. For any atom or molecule, the main goal is to attain stability by attaining the noble gas configuration. So, they tend to share or transfer electrons to become stable. This acts as the main driving force for the atoms to combine with one another.
Conclusion
Chemical bonding or chemical combination is the process by which two or more atoms combine to form a chemical compound. The main reason why they tend to combine with one another is to obtain the stable noble gas electron configuration. This is done by the atoms either by losing or gaining electrons or by mutually sharing electrons between other atoms.
There are different types of chemical bonds that can be formed between atoms based on their strength and properties. Which of the atoms will attain extra stability depends on the type of bond. Ionic and covalent bonds are strong, whereas hydrogen bonds and Van der Waals interactions are comparatively weak forces of attraction between the atoms.
FAQs on Causes of Chemical Bonding for JEE
1. What causes nitrogen to be chemically inert?
Nitrogen is usually considered to be less reactive or chemically inert at room temperature. The main reason for this is the presence of three strong bonds or triple bonds between the 2 atoms of nitrogen present within a nitrogen molecule. In addition to this, their small atomic size also draws them further closer to one another. So in order to break such three strong bonds, really high energy would be required. This is not a favourable reaction and hence, they are preferably chemically inert.
2. An atom is electrically neutral. Give reasons.
The constituents of an atom are protons, neutrons, and electrons. These are known as subatomic particles. The protons are positively charged whereas the electrons are negatively charged. The neutrons have no charge and hence are neutral. The nucleus consists of neutrons and protons. The electrons are found revolving around the atom in its orbits. The total number of protons is always equal to the tidal number of electrons. Hence, the overall charge of an atom is zero, making an atom electrically neutral.
























