




Learn About Coordination Compound Definition, Types, Nomenclature And Applications
Coordination Compounds are a vital aspect of Chemistry, involving a central metal atom or ion bonded to surrounding molecules or ions called ligands. These complexes play significant roles in catalysis, biological processes, material science, and industrial applications. This content offers a detailed yet concise overview of the definition, bonding theories, isomerism, nomenclature, and real-world applications of coordination compounds. Whether you're preparing for competitive exams or enhancing your knowledge of inorganic chemistry, this guide ensures you grasp every essential concept.
What is Coordination Compound?
A coordination compound is a type of chemical compound that consists of a central metal atom or ion surrounded by molecules or ions called ligands. These ligands are bonded to the central metal atom or ion through coordinate covalent bonds, where the ligand donates a pair of electrons to the metal.
Coordination compounds play an essential role in various chemical, biological, and industrial processes. Examples include haemoglobin, chlorophyll, and catalysts used in industrial reactions.
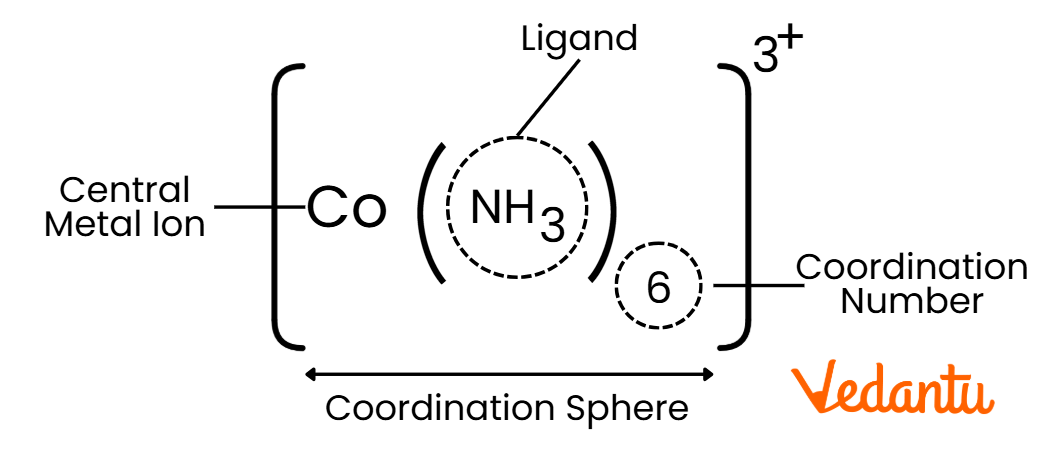
Key Components of Coordination Compounds:
Central Metal Atom/Ion: Usually a transition metal that acts as the core of the compound.
Ligands: Molecules or ions that donate electron pairs to the central metal (e.g., water, ammonia, chloride ions).
Coordination Number: The number of ligand bonds around the central metal atom.
Coordination Sphere: The central metal and the ligands directly attached to it.
Example:
[CO(NH)3]3+ : A coordination compound where iron (CO) is the central metal, and cyanide (NH3) acts as the ligand.
Werner’s Theory of Coordination Compounds
Alfred Werner's theory provides a fundamental understanding of coordination compounds. The main concepts include:
Primary valency satisfies the oxidation state of the metal and is ionizable.
Example: In $[Co(NH_3)_6]Cl_3$, the primary valency is $3$, represented by the three chloride ions.Secondary valency represents the coordination number and is fulfilled by ligands.
Example: In $[Co(NH_3)_6]Cl_3$, the secondary valency is $6$, represented by six ammonia molecules.
The ligands arrange themselves in specific geometries, such as octahedral, tetrahedral, or square planar.
2. Isomerism in Coordination Compounds
Isomerism in coordination compounds arises when compounds have the same molecular formula but differ in structure or spatial arrangement.
In coordination isomerism, ligands exchange between cationic and anionic parts:
$[Co(NH_3)_6][Cr(CN)_6]$ and $[Cr(NH_3)_6][Co(CN)_6]$.
Ionization isomerism occurs when ligands and counter ions interchange positions:
$[Co(NH_3)_5SO_4]Br$ and $[Co(NH_3)_5Br]SO_4$.Linkage isomerism arises in ambidentate ligands, where binding occurs through different donor atoms:
$[Co(NH_3)_5(NO_2)]^{2+}$ (Nitro) and $[Co(NH_3)_5(ONO)]^{2+}$ (Nitrito).Solvate isomerism occurs when water acts either as a ligand or as a free molecule:
$[Cr(H_2O)_6]Cl_3$ and $[Cr(H_2O)_5Cl]Cl_2 \cdot H_2O$.Geometrical isomerism occurs due to differences in the spatial arrangement of ligands.
Example: In $[Pt(NH_3)_2Cl_2]$, the cis isomer has adjacent ligands, while the trans isomer has ligands opposite each other.Optical isomerism occurs in non-superimposable mirror images (enantiomers).
Example: $[Co(en)_3]^{3+}$.
3. IUPAC Nomenclature of Coordination Compounds
The systematic rules for naming coordination compounds are:
Write ligand names alphabetically before the central metal.
Use prefixes such as di-, tri- for monodentate ligands and bis-, tris- for polydentate ligands. Anionic ligand names end with "o" (e.g., chloride → chlorido).
The oxidation state of the central metal is indicated in Roman numerals.
Example: $[Co(NH_3)_6]Cl_3$ is named Hexaamminecobalt(III) chloride.
4. Bonding in Coordination Compounds
Valence Bond Theory (VBT) explains bonding through the overlap of the metal’s vacant orbitals with the ligand’s filled orbitals. Hybridization determines geometry. For an octahedral complex:
$d^2sp^3$.Crystal Field Theory (CFT) explains the splitting of metal d-orbitals when ligands approach. For an octahedral field:
$d$-orbitals split into $t_{2g}$ (lower energy) and $e_g$ (higher energy).
The energy difference ($\Delta$) determines color (via light absorption) and magnetism (via high-spin or low-spin configurations depending on ligand strength).
5. Ligands and Coordination Number
Ligands donate lone pairs to the metal. Examples include monodentate ($NH_3, Cl^-$), bidentate (ethylenediamine, $en$), and polydentate (EDTA).
The coordination number refers to the number of ligand donor atoms attached to the metal.
Examples:
$CN = 4$: Tetrahedral ($[Ni(CN)_4]^{2-}$).
$CN = 6$: Octahedral ($[Fe(CN)_6]^{3-}$).
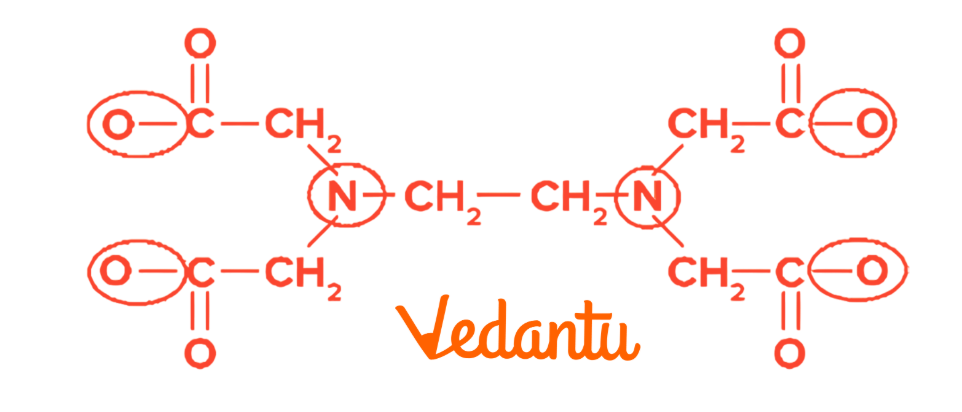
6. Chelation
Chelation occurs when polydentate ligands form stable cyclic structures with metals.
EDTA is a common chelating agent used in medicine to treat heavy metal poisoning and in environmental science to remove toxic metal ions from water.
There are 6 Donor sites in EDTA .
7. Applications of Coordination Compounds
Coordination compounds play significant roles in various fields. Industrially, they are used in catalysis (e.g., the Haber process for ammonia synthesis), electroplating, and dye production.
Biologically, hemoglobin ($Fe$) transports oxygen, and vitamin B12 ($Co$) aids metabolism.
Medicinally, cisplatin is used in cancer treatment. In environmental science, coordination compounds help in pollution control and water purification.
Conclusion
Coordination compounds are foundational to inorganic chemistry, bridging theoretical principles with practical applications. Their role in catalysis, biological systems, and industrial processes underscores their importance. This structured guide ensures a comprehensive understanding of these fascinating compounds.
What is Coordination Compounds?

 Share
ShareFAQs on What is Coordination Compounds?
1. What are coordination compounds?
Coordination compounds are chemical compounds where a central metal atom or ion is surrounded by ligands that form coordinate covalent bonds. These compounds are important in biological, industrial, and medicinal fields.
2. What does coordination compounds class 12 topics cover?
Coordination compounds class 12 covers topics such as Werner’s theory, nomenclature, isomerism, bonding theories (VBT and CFT), stability of coordination compounds, and their applications in daily life.
3. Where can I download the coordination compounds NCERT PDF?
The coordination compounds NCERT PDF for class 12 chemistry can be downloaded from Vedantu website.
4. What are the applications of coordination compounds?
Applications of coordination compounds include their use in industrial catalysis (e.g., Haber process), medicine (e.g., cisplatin for cancer treatment), biological systems (e.g., hemoglobin for oxygen transport), and environmental science (e.g., chelation therapy for heavy metal poisoning).
5. What is the nomenclature of coordination compounds?
The nomenclature of coordination compounds follows IUPAC rules, where ligands are named first in alphabetical order, followed by the central metal ion with its oxidation state in Roman numerals. For example, $[Co(NH_3)_6]Cl_3$ is named Hexaamminecobalt(III) chloride.
6. What is coordination compound?
A coordination compound consists of a central metal atom or ion bonded to ligands that donate lone pairs of electrons. These compounds have unique properties, such as variable geometry and color.
7. What is the coordination compounds definition?
The definition of coordination compounds states that they are compounds formed by a central metal atom or ion surrounded by ligands, which are neutral molecules or anions capable of donating electron pairs.
8. What is Werner theory of coordination compounds?
Werner’s theory explains the bonding in coordination compounds. It states that the central metal atom exhibits primary (oxidation state) and secondary (coordination number) valencies, determining the compound's composition and geometry.
9. How are coordination compounds used in Medicine and Biology?
Coordination compounds play a vital role in medicine and biology. Examples include cisplatin, used in cancer treatment, and haemoglobin, a coordination compound responsible for oxygen transport in the body.
10. How can I understand coordination compounds class 12 easily?
To understand coordination compounds class 12, focus on the NCERT textbook for clear explanations of Werner’s theory, bonding, isomerism, and applications. Solve exercises and refer to diagrams for better conceptual clarity.




















 Watch Video
Watch Video











