




Hydrocarbons: A Brief Introduction
Hydrocarbons are compounds that only contain carbon and hydrogen. They are primarily derived from petroleum, natural gases, and other sources. There are three types of hydrocarbons based on the type of carbon-carbon bond present: saturated, unsaturated, and aromatic hydrocarbons. Saturated hydrocarbons have only single carbon-carbon bonds. Alkanes and cycloalkanes are two examples. Unsaturated hydrocarbons have multiple carbon-carbon bonds, such as alkenes and alkynes. Aromatic hydrocarbons contain at least one type of hexagonal ring with a six-carbon atom and three double bonds in the alternate position, such as the benzene ring. With examples, we will concentrate on cycloalkanes and allenes in this article.
Cycloalkanes
Carbocyclic hydrocarbons are those in which carbon atoms are joined by single covalent bonds to form a closed ring of carbon atoms. Cycloalkanes are saturated hydrocarbons. They are alicyclic. The formula of cycloalkanes with one ring is CnH2n, whereas acyclic alkanes have the general formula CnH2n+2. As another carbon-carbon bond is needed to form the ring, cycloalkanes have two fewer hydrogen atoms than alkanes. Cycloalkanes are not aromatic.
Structure of cycloalkane; The structure of cycloalkane contains a single ring made of carbon atoms, including all carbon-carbon bonds being single. Carbon's free valencies are satisfied by hydrogen atoms.
Stability of Cycloalkane
A. Baeyer, a well-known German organic chemist, proposed in 1890 that cyclopropane and cyclobutane are less stable than cyclohexane because the smaller rings are more "strained." Angle strain, torsional strain, and steric strain are all types of strain that contribute to the overall ring strain in cycloalkanes.
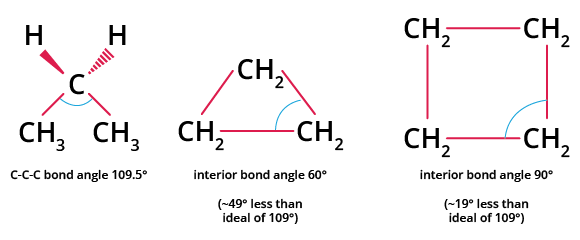
Deviation in the Bond Angle of Cyclopropane and Cyclobutane
Angle strain occurs when cycloalkane sp3 hybridised carbons do not have the expected ideal bond angle of $109.5^{\circ}$, leading to an increase in potential energy.
The C-C-C bond angles in cyclopropane (shown above) $60^{\circ}$ and cyclobutane $90^{\circ}$ differ markedly from the ideal bond angle of $109.5^{\circ}$. As the bond angle between the carbons is much closer to $109.5^{\circ}$, cyclopropane and cyclobutane are less stable than molecules like cyclohexane and cyclopentane, which have a much lower ring strain because the bond angle between the carbons is much closer to $109.5^{\circ}$. In addition to angle strain, many cycloalkanes have steric (transannular) strain and torsional strain. When there is steric repulsion between atoms, transannular strain exists.
A torsional (eclipsing) strain exists when a cycloalkane cannot adopt a staggered conformation around a C-C bond due to an inability to rotate freely. Torsional strain is especially common in small cycloalkanes with nearly planar structures, such as cyclopropane.
Larger rings, such as cyclohexane, deal with torsional strain by forming conformers with non-planar rings. Conformers are stereoisomer molecules that exist as different isomers of the same connectivity and formula, in this case, to reduce ring strain. Conformers have less ring strain because the rotations around the sigma bonds reduce the angle and torsional strain in the ring. Cyclohexane's non-planar structures are much more stable than those of cyclopropane and cyclobutane.
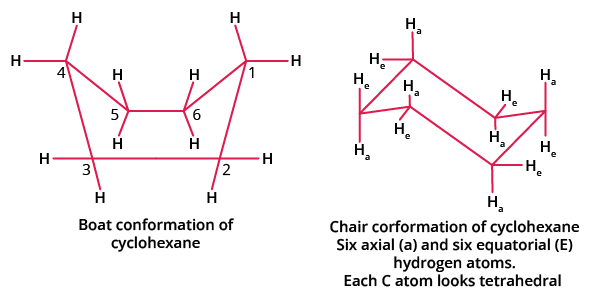
Stability of Cyclohexane
Examples of cycloalkanes include cyclopropane, cyclobutane, cyclopentane, and cyclohexane as shown in the image below. The structure of cycloalkane is determined by the number of carbon atoms present in the compound.
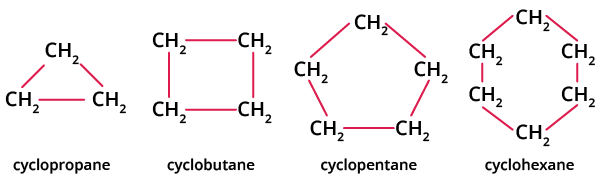
Examples of Cycloalkanes
Allenes
Allenes is a "cumulated dienes." Dienes alkenes contain both sigma( σ) bonding formed by "head-on" orbital overlap (i.e. "single bonds) and pi (π) bonding formed by "side-on" orbital overlap (i.e.multiple bonds). Allene belongs to the cumulene family of molecules because the double bonds are cumulative (consecutive). Allene's central carbon is sp hybridised.
The allene structure contains three carbon atoms and two double bonds between them. Allene's left half is perpendicular to its right half. The two-terminal carbon atoms are planar and twisted 90 degrees from each other. The structure is also known as an "extended tetrahedral," and it has a similar shape to methane. It is achiral if either of those ends is connected to two identical substituents – because it has a mirror plane and vice-versa.
Optical Activity of Allenes
Chirality causes optical activity. Chirality occurs when one molecule cannot be superimposed on its mirror image. Since there is no rotation about the double-bonded carbons in an allene with four different substituents, the mirror image of itself is not superimposable; it is chiral.
Allenes with any number of pi bonds are optically active if the groups on the carbon are not the same. This is due to the molecule's lack of plane of symmetry and centre of symmetry. In other words, Allenes with an even number of the carbon atoms are optically inactive due to the presence of symmetry whereas allenes with an odd number of the carbon atoms are optically active because of the absence of symmetry in the molecules.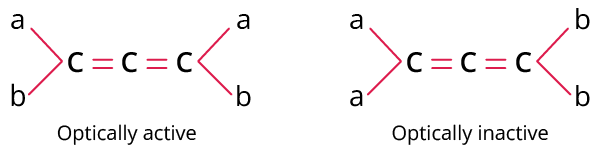
Optical Activity of Allenes
Allenes Examples
Chiral allenes are those allenes which have two different substituents on each of the two carbon atoms, and thus lack a mirror plane.
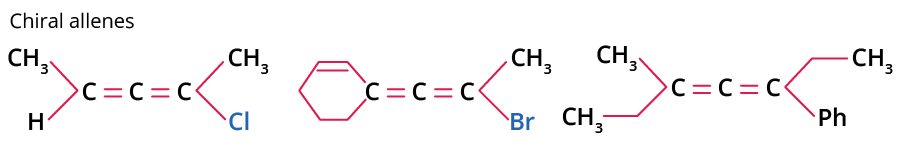
A Few Examples of Chiral Allenes
Conclusion
Cycloalkane are the type of hydrocarbons in which each carbon is bonded to the next carbon by a single bond. Cycloalkane is a saturated cyclic compound. The nomenclature of cycloalkane can depend upon the number of carbon atoms present in the ring. For example, cyclopropane, cyclobutane, cyclopentane and cyclohexane, and so on. Allenes can be defined as the cumulated dienes. Allenes are optically active compounds due to the absence of symmetry i.e. p-orbital of one carbon atom is perpendicular to the p-orbital of another carbon atom. In other words, we can say that allenes are optically active when they contain odd numbers of carbon atoms.
FAQs on Cycloalkanes and Allenes with Examples - JEE Important Topic
1. What is the significance of cycloalkanes in Chemistry?
There are some applications of cycloalkane in Chemistry which are listed below:
Cycloalkanes are used as an organic solvent in the production of drugs in medical applications.
Carboplatin, derived from cyclobutane, is used to treat cancer. They are also employed in the oil and gas industries.
Some cycloalkane classes are used for pigmentation and as fragrances in the perfume manufacturing industry.
Some of these saturated hydrocarbons are found as steroids in plant and animal tissues. These are used in the production of hair products as well as in the food industry.
In the medical field, cycloalkane cyclopropane is used as an anaesthetic agent.
2. What exactly are cumulene and allene and how are they related to each other?
A cumulene is a hydrocarbon that contains three or more cumulative (concurrent) double bonds. They are similar to allenes but have a longer chain. Butadiene $(H_2C=C=C=CH_2)$ known as cumulene is the simplest molecule in this class.
Allene and cumulene are both organic compounds. These are alkenes with double carbon-atom bonds. Allene has three carbon atoms and two double bonds between them. Cumulene, on the other hand, has four carbon atoms connected by three double bonds. Allenes show optical isomerism whereas cumulene is optically inactive.
3. Is geometrical isomerism present in allenes?
Geometrical isomerism is a kind of stereoisomerism wherein the molecular formula and structure are the same but atoms are arranged differently. To determine whether a molecule has geometrical isomerism, it must have restricted rotation involving a carbon-carbon double bond. There should be two distinct compounds on the left and right sides of the double bond.
Due to the geometry of this bond, the groups attached to the terminal C atom lie in perpendicular planes. These chiral allenes have different substituents on end C. Such allenes exhibit optical activity rather than cis-trans isomerism.
























