




What is the Balanced Chemical Equation for the Hall-Héroult Process?
The Hall Heroult Process with Reaction is the principal technique for extracting aluminium from its oxide, alumina, using high-temperature electrolysis. This process revolutionised the production of aluminium, making it accessible for widespread use. Understanding the Hall Heroult mechanism, its balanced reaction, diagrammatic setup, and the industrial importance is essential for JEE Main Chemistry preparation.
What is the Hall Heroult Process?
The Hall Heroult process is an electrochemical method used to extract aluminium metal from molten alumina (Al2O3). This is achieved by dissolving alumina in molten cryolite (Na3AlF6), lowering the melting point, and then applying an electric current. Both the reaction equation for the Hall Heroult process and its stepwise procedure are targeted frequently in JEE exams. This method is distinct from the Bayer process, which is used to purify bauxite before electrolysis.
Balanced Chemical Reaction and Equation
The key Hall-Héroult process chemical reaction is:
| Overall Reaction | Cathode (Reduction) | Anode (Oxidation) |
|---|---|---|
| 2Al2O3 + 3C → 4Al + 3CO2 | Al3+(melt) + 3e- → Al(l) | C(s) + 2O2-(melt) → CO2(g) + 4e- |
Here, Al2O3 (alumina) is electrolysed using cryolite as solvent. Graphite acts as both the anode and cathode. Oxygen released at the anode reacts with graphite to form CO2. The metal aluminium is deposited at the cathode.
Step-by-Step Process of the Hall Heroult Extraction
- Bayer process: Purify bauxite ore to obtain pure alumina.
- Dissolve alumina in molten cryolite (Na3AlF6) and add a small amount of CaF2 to lower melting point (about 950–1000 °C).
- Set up the iron tank lined with graphite as the cathode; insert carbon (graphite) rods as the anode.
- Maintain the bath at 1173 K (900 °C). Ensure the mixture remains molten for continuous electrolysis.
- Pass a high electric current through the cell. Aluminium ions move to the cathode (reduce to Al), oxide ions move to anode (form CO2).
- Molten aluminium is denser and settles at the bottom; periodically tapped off from the cell.
- Replace the consumed graphite anodes regularly as they are oxidised to CO2.
Hall Heroult Process Diagram and Cell Setup
A clear diagram of the Hall Heroult process cell highlights the position of the graphite anode and cathode, the molten electrolyte (alumina + cryolite), and paths for aluminium collection and gas escape. For JEE, proper labelling and structural understanding are essential.
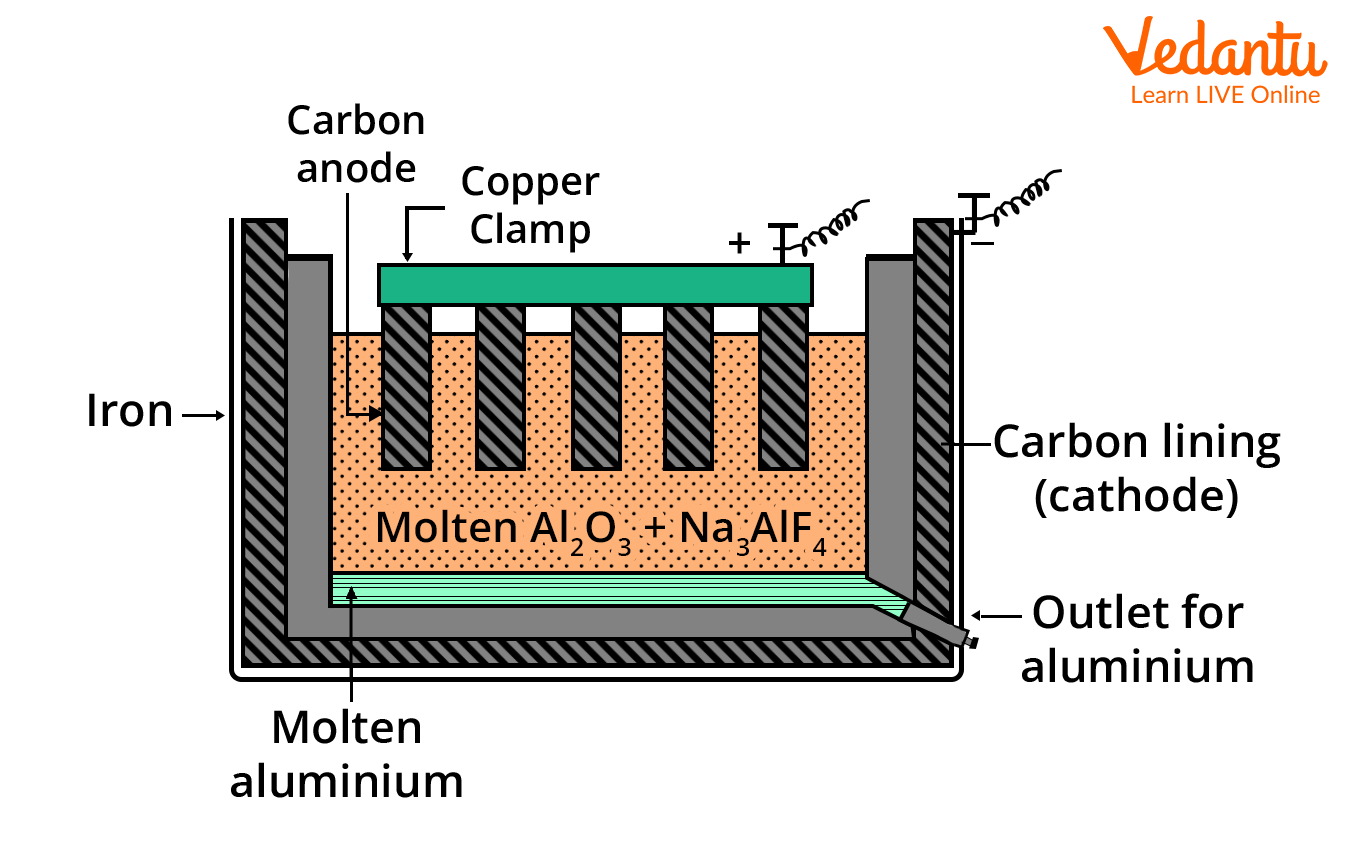
| Part | Description |
|---|---|
| Anode | Thick graphite rods (consumed during reaction, produce CO2) |
| Cathode | Graphite lining of the iron tank (where aluminium forms) |
| Electrolyte | Fused mixture of alumina, cryolite, and a little fluorspar (CaF2) |
| Tapped Product | Molten aluminium collected at the cell bottom |
Industrial Importance and Applications
The Hall Heroult process is the only economically viable method for large-scale aluminium extraction. It provides aluminium of up to 99.95% purity, making it invaluable for the electrical, automotive, construction, and packaging industries. The process outperforms older chemical reduction techniques due to efficiency, lower operating temperature (thanks to cryolite), and a high degree of metal purity.
- Aluminium extracted is used for cables, utensils, transport parts, and building materials.
- Compared to the Bayer process, which provides purified Al2O3, the Hall Heroult directly gives metallic aluminium.
- Regular replacement of graphite anode adds to operating cost, but process remains unmatched for volume and purity.
Solved JEE Example and Application Practice
Example: Calculate the amount (in moles) of aluminium deposited when a current of 96,500 C passes through the Hall Heroult cell.
- The cathode reaction: Al3+ + 3e- → Al
- 1 mole Al requires 3 × 96,500 C (Faraday's law).
- Moles of Al = total charge / (3 × 96,500).
- Moles Al = 96,500 / 289,500 = 0.333 moles (rounded to three decimals).
Always check the number of electrons involved and use Faraday's constant when solving aluminium extraction or electrolysis numericals for JEE practice.
Key Differences: Hall Heroult vs Bayer Process
| Feature | Hall Heroult Process | Bayer Process |
|---|---|---|
| Purpose | Extraction of aluminium metal | Purification of bauxite ore |
| Type | Electrolytic reduction | Chemical extraction |
| Main Chemicals | Al2O3, cryolite, graphite | NaOH, bauxite, water |
| End Product | Metallic aluminium (99.95% pure) | Pure alumina (Al2O3) |
Fast Facts and Exam Tips
- Cryolite increases conductivity and lowers the melting point of alumina.
- Both anode and cathode are made of graphite, but the anode is consumed over time due to oxidation.
- Use Faraday’s laws of electrolysis for quantitative numericals.
- Draw and label the Hall Heroult cell precisely in answers.
- The process operates just under 1000 °C; avoid confusing with Bayer process conditions.
Mastering the Hall Heroult Process with Reaction gives a sharp edge in the JEE Main Chemistry syllabus. For more redox and electrolysis fundamentals, check Redox Reactions and Electrolysis resources. Structural clarity and numerical skill are essential to ace related JEE questions, and Vedantu’s deep coverage can bolster your preparation.
FAQs on Hall-Héroult Process with Reaction, Steps, and Diagram
1. What is the Hall-Héroult process in chemistry?
The Hall-Héroult process is the primary industrial method for extracting aluminium from its oxide, alumina (Al₂O₃), using electrolytic reduction. The process uses a specially designed electrolysis cell with a molten mixture of alumina and cryolite to efficiently produce pure aluminium metal.
Key features include:
- Utilises a molten electrolyte of alumina dissolved in cryolite.
- Employs graphite electrodes as anode and cathode.
- Widely used for industrial production of aluminium worldwide.
2. What is the chemical reaction of the Hall-Héroult process?
The main chemical reaction in the Hall-Héroult process is the electrolytic reduction of alumina to aluminium metal using carbon electrodes.
The balanced equation:
2Al₂O₃ (l) + 3C (s) → 4Al (l) + 3CO₂ (g)
- Al₂O₃ gets reduced at the cathode
- Carbon (graphite) at the anode is oxidised to carbon dioxide
- Pure aluminium settles at the bottom of the cell
3. What are the steps of the Hall process?
The Hall-Héroult process involves several key steps for extracting aluminium from alumina:
1. Purification of bauxite to obtain pure alumina (often by the Bayer process).
2. Dissolving alumina in molten cryolite (Na₃AlF₆) to lower its melting point.
3. Electrolysis in a specially designed steel cell lined with graphite.
4. Reduction of aluminium ions at the cathode to form liquid aluminium.
5. Oxidation of carbon anode to form carbon dioxide gas.
6. Periodic removal of produced molten aluminium from the cell.
4. What is the equation for the Hall-Héroult process?
The equation for the Hall-Héroult process summarises the conversion of alumina and carbon into aluminium and carbon dioxide:
2Al₂O₃ + 3C → 4Al + 3CO₂
This reaction takes place in a molten cryolite-based electrolyte during the electrolysis of alumina.
5. Why is cryolite used in the Hall-Héroult process?
Cryolite (Na₃AlF₆) is used in the Hall-Héroult process to:
- Lower the melting point of alumina from around 2072°C to about 950–1000°C, making the process more energy efficient.
- Increase the conductivity of the electrolyte, enabling better flow of electric current.
- Allow alumina to dissolve properly for efficient electrolysis.
6. What are the conditions for the Hall-Héroult process?
The typical conditions required for the Hall-Héroult process are:
- Electrolyte: Mixture of alumina and molten cryolite
- Temperature: 950–1000°C
- Anode and cathode: Both made of carbon (graphite), the cell itself acts as cathode
- Continuous addition of alumina as it gets consumed
- Direct current supply: For sustained electrolysis
7. What is the role of graphite electrodes in the Hall process?
Graphite electrodes serve critical functions in the Hall-Héroult process:
- Cathode: The cell lining (graphite) collects the produced aluminium metal.
- Anode: Graphite rods supply electrons and react with oxygen to form carbon dioxide gas.
- This sacrificial anode needs periodic replacement as it gets oxidised and consumed.
8. How is aluminium separated from the electrolyte in the Hall-Héroult process?
In the Hall-Héroult process, aluminium metal forms at the cathode where it collects at the bottom of the electrolytic cell as a molten layer. It is then periodically tapped off for further purification or casting.
- Aluminium is denser than molten electrolyte, allowing it to settle at the base.
- The process enables continuous extraction during electrolysis.
9. What happens to the graphite anode during the Hall-Héroult process?
During the Hall-Héroult process, the graphite anode undergoes oxidation by reacting with oxygen ions produced at the anode, forming carbon dioxide gas (CO₂).
- As a result, the graphite anode is gradually consumed.
- Regular replacement of the anode is necessary for continuous operation.
10. How is the Hall-Héroult process different from the Bayer process?
The Hall-Héroult process and Bayer process are distinct stages in aluminium metallurgy:
- Bayer process: Used to purify bauxite ore to produce pure alumina (Al₂O₃).
- Hall-Héroult process: Uses purified alumina to extract metallic aluminium via electrolysis.
- The Hall-Héroult process comes after the Bayer process in the industrial sequence.























