




IUPAC Nomenclature of Alkanes
A systematic way of naming hydrocarbons and other organic compounds has been implemented by the International Union of Pure and Applied Chemistry (IUPAC). These rules are used worldwide and are known as the IUPAC System of Nomenclature.All alkanes have names that end in -ane. Every carbon-hydrogen chain that lacks any double bonds or functional groups is referred to by the suffix -ane, regardless of whether the carbons are linked end-to-end in a ring (in which case they are known as cyclic alkanes or cycloalkanes) or whether they have side chains and branches. The number of carbons in an unbranched carbon chain is used to name alkanes. To distinguish straight-chain alkanes from branched-chain alkanes with the same number of carbon atoms, the prefix n- (for normal) is sometimes used. Although not strictly necessary, this is still used when there is a significant difference in properties between the straight-chain and branched-chain isomers.
What are Alkanes?
Alkanes are hydrocarbons that contain only one single bond in them. The first four members of the series are known by their common names, that is, Methane, Ethane, Propane, and Butane, respectively. The names of the larger alkanes are derived from the Greek prefixes that indicate the number of carbon atoms in the molecule. Thus, pentane has 5 carbons, hexane has 6 carbons, and so on.
In the common system, all the isomeric alkanes have the same parent name. For example, when we consider the two isomeric C4H10 alkanes that are known as butanes. The names of various isomers are distinguished by their prefixes. The prefix indicates the type of branching that is present in the molecule.
List of Names of First 10 Straight Chain Alkanes
1. Meaning of Prefix n-
The prefix n- is used for those alkanes whose all carbons atoms are in one continuous chain. The prefix n- generally stands for a normal or straight chain.
Eg: CH3CH2CH2CH3 - n- Butane, CH3CH2CH2CH2CH3 - n- Pentane.
2. Meaning of the Prefix Iso-
The prefix iso- is used for those alkanes which have a methyl group (CH3–) attached to the second last carbon of the continuous chain.
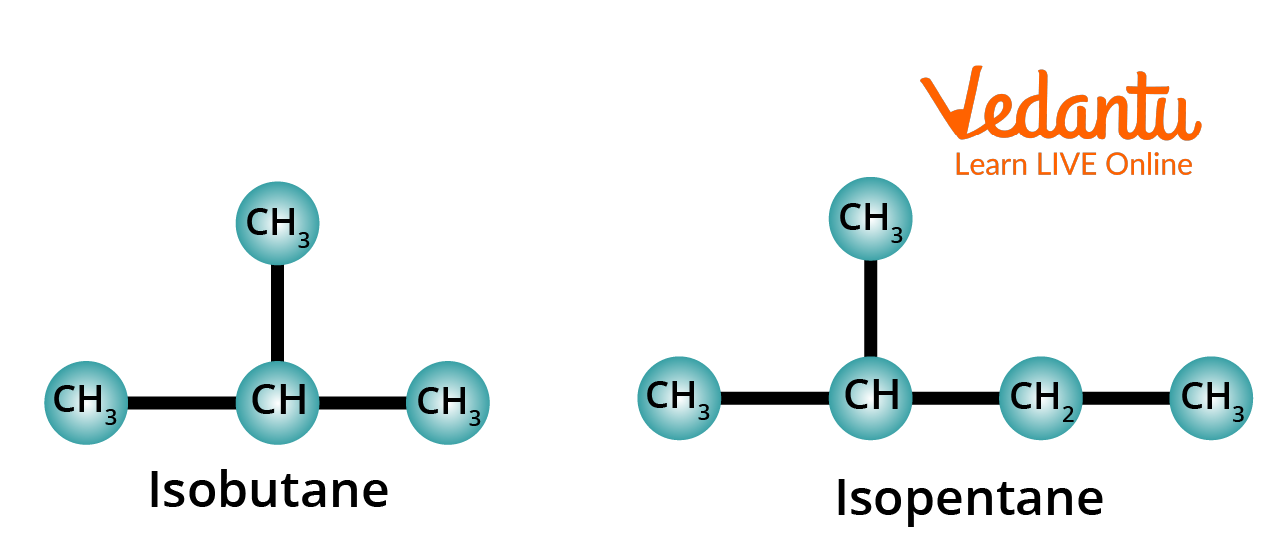
Structure of Isobutane and Isopentane
3. Meaning of the Prefix Neo-
The prefix neo- is used for those alkanes which have two methyl groups attached to the second last carbon atom of the continuous chain.
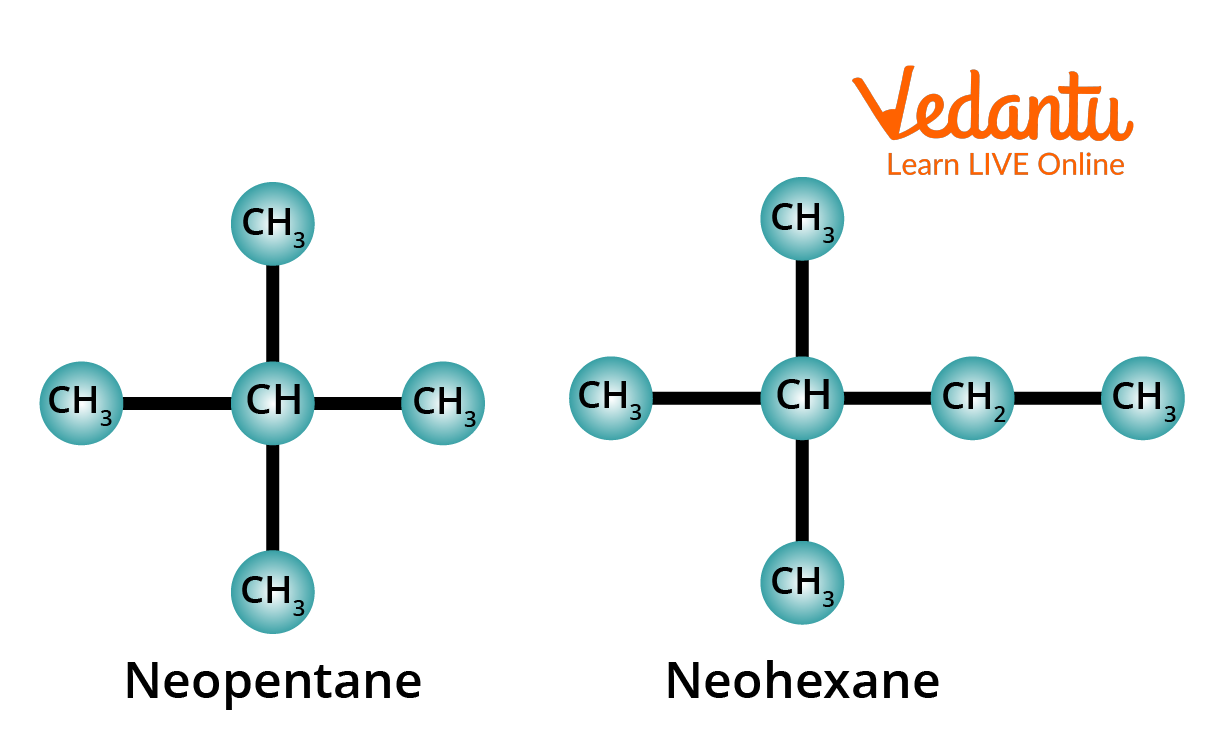
Structure of Neopentane and Neohexane
Classification of Carbon Atoms
The structural formulas of alkanes contain four types of carbons:
1. Primary Carbon: A carbon atom attached to one another (or no other) carbon atom is called Primary carbon. (1∘ Carbon: 1∘ = Primary).
2. Secondary Carbon: A carbon atom that is attached to two other carbon atoms is called Secondary carbon. (2∘ Carbon: 2∘ = Secondary).
3. Tertiary Carbon: A carbon atom that is attached to three other carbon atoms is called Tertiary carbon. (3∘ Carbon: 3∘ = Tertiary).
4. Quaternary Carbon: A carbon atom that is attached to four other carbon atoms is called a Quaternary carbon. (4∘ Carbon: 4∘ = Quaternary).
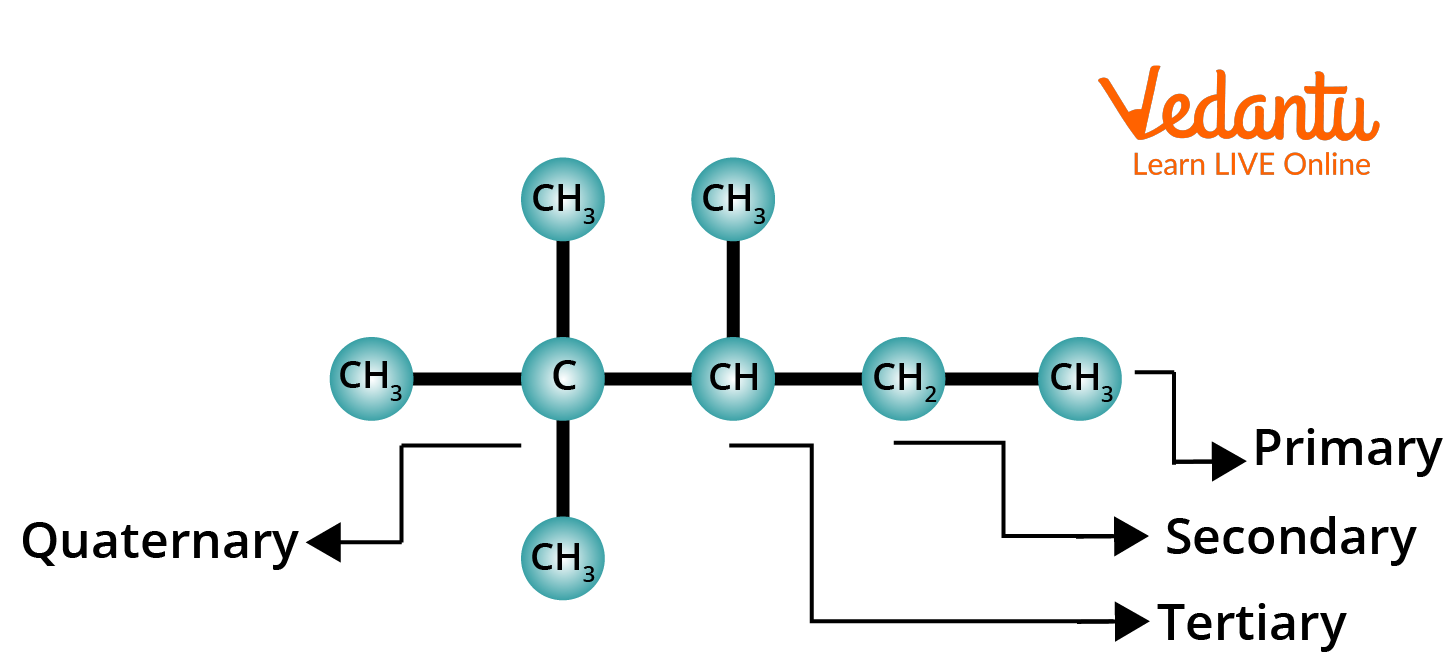
Primary, Secondary, Tertiary and Quaternary Alkanes
Alkyl and Non-Alkyl Groups
Alkyl Groups
An Alkyl group is formed by removing one of the hydrogen atoms from an alkane.
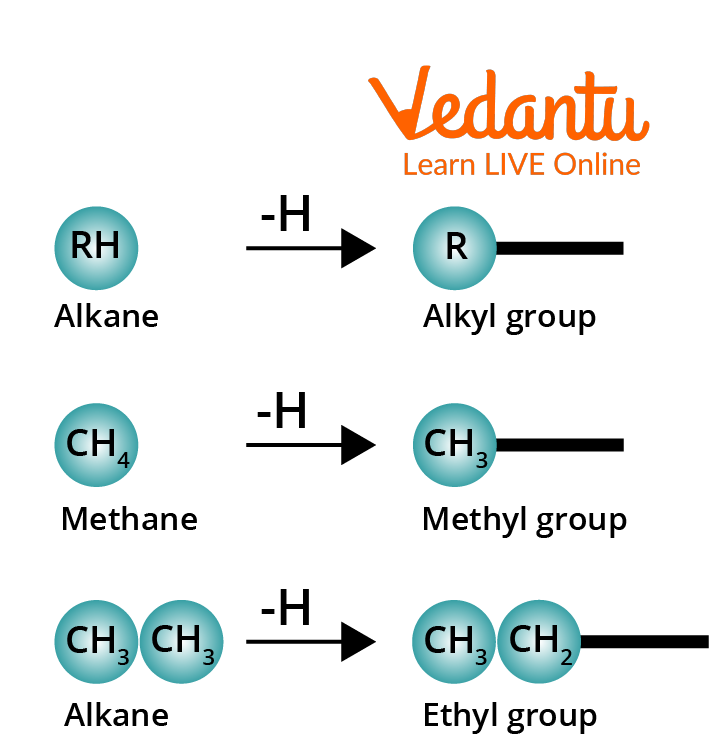
Conversion of Alkane to an Alkyl Group
The symbol R– is prevalently used to represent an alkyl group. The grouping R– (eg., CH3CH2CH2–) is not a compound and it should be bonded to another atom or a group of atoms. Alkyl groups are named by dropping the suffix -ane from the name of the corresponding alkane and adding -yl in the place of the suffix -ane.
Non-alkyl Groups
A number of non-alkyl groups are used in naming the organic compounds. Their names are given below:
-Cl = Chloro
-Br = Bromo
-I = Iodo
-F = Fluoro
-NO2 = Nitro
-NO = Nitroso
-NH2 = Amino
-OH = Hydroxy
IUPAC Rules for Naming Alkanes
The IUPAC system is much the same for all classes of organic compounds. The IUPAC rules for naming the alkanes are given below. Master them!
Rule 1: Select the longest continuous carbon chain. Remember that this chain does not have to be the portion of the molecule that is written horizontally. This is a mistake that is made by many beginners.
Rule 2: Name the longest chain. The longest carbon chain is selected on the basis of the name. The names are given in the above table. These are considered the parent names.

Structure of Butane
The longest continuous chain has four carbon atoms. Thus, the compound name is Butane.
Rule 3: Number the longest chain. The carbon atoms present in the longest chain are numbered. The numbering starts from that end so that it will give numbers that are having the lowest value to carbons carrying substituents.
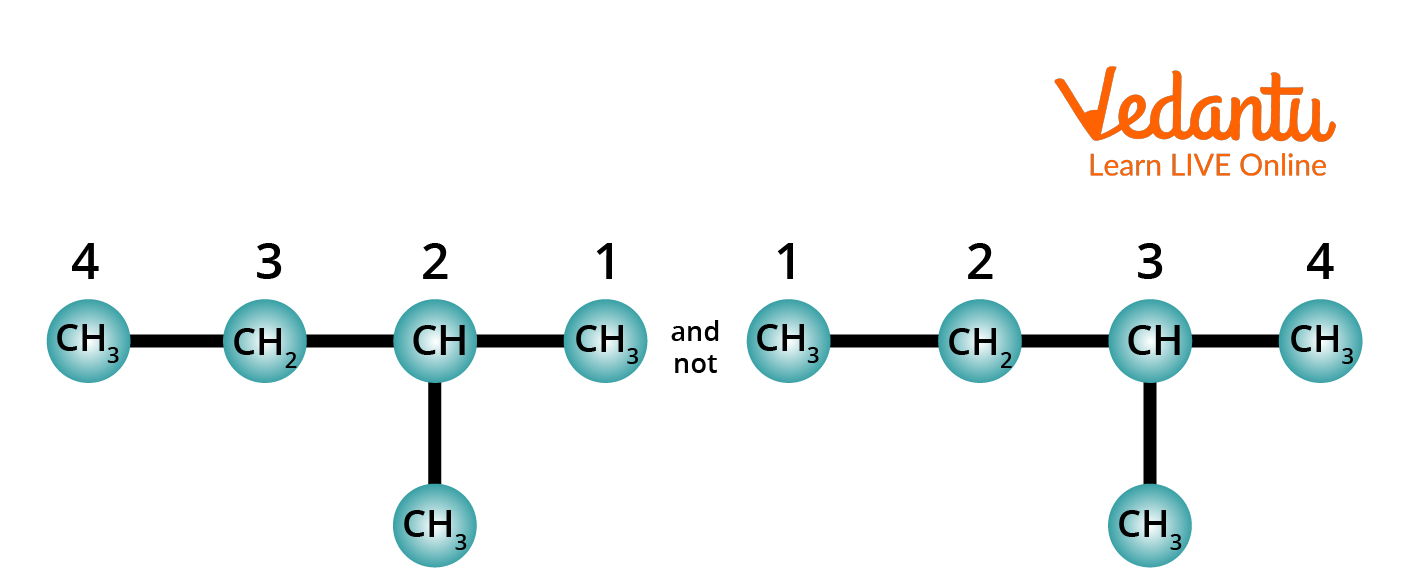
Method of Numbering Carbon Chain
Rule 4: Identify the substituent. Name the substituent. Indicate its position by the number of carbon-carrying substituents.
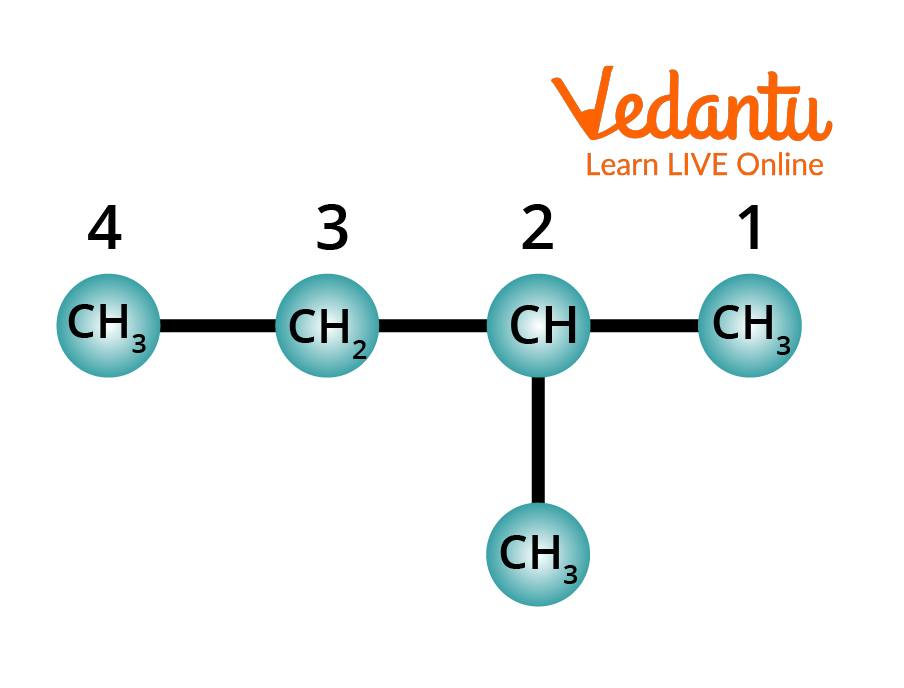
Identifying the Substituent in Alkane
The attached group is located on carbon 2 of the long chain, and it is a methyl (CH3) group.
Rule 5: Prefix the position number and name the substituent onto the parent name of the alkane. The whole name is written as one word. You must note that the number and the name of the substituent in the chain are separated by a hyphen.
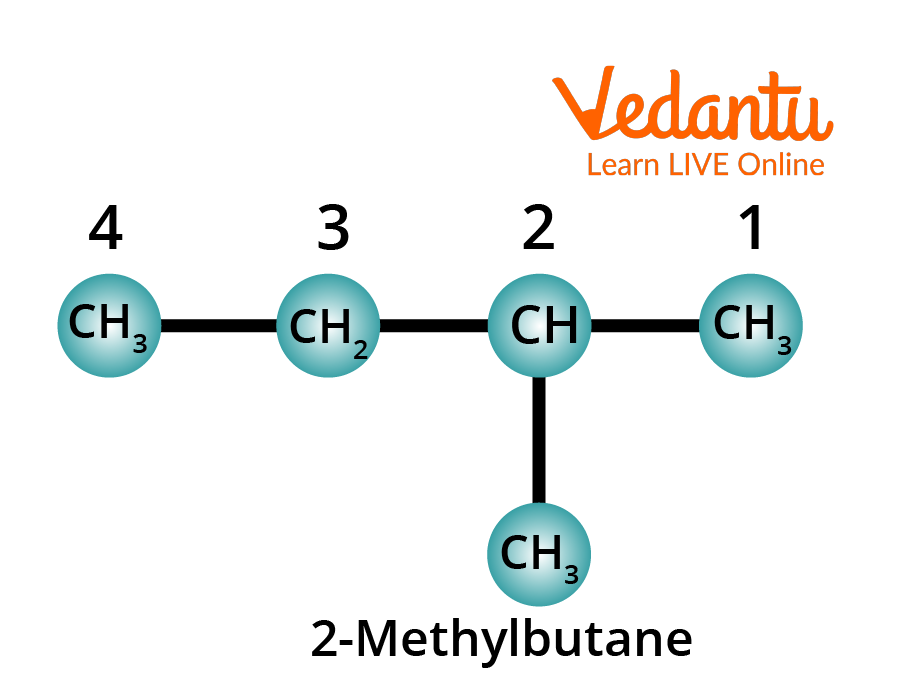
Structure of 2-Methylbutane
Rule 6: Identify the substituents by names and position numbers in the chain. When the same substituent is present two or more times in the same molecule, prefixes such as di-, tri-, tetra-, penta-, hexa-, etc. are used. The position of each substituent in the carbon chain is indicated using a separate number. These position numbers, separated by a comma, are put just before the name of the substituent, with a hyphen before and after the numbers when necessary.
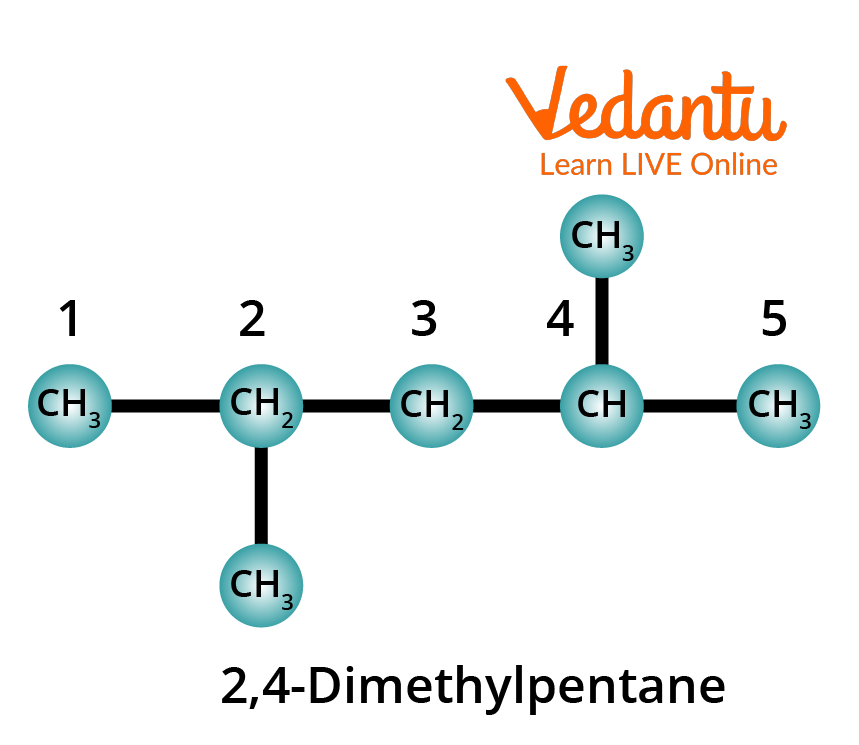
Rule 7: When two or more different substituents are present, their names are arranged in alphabetical order and added to the name of the parent alkane, again as one word.
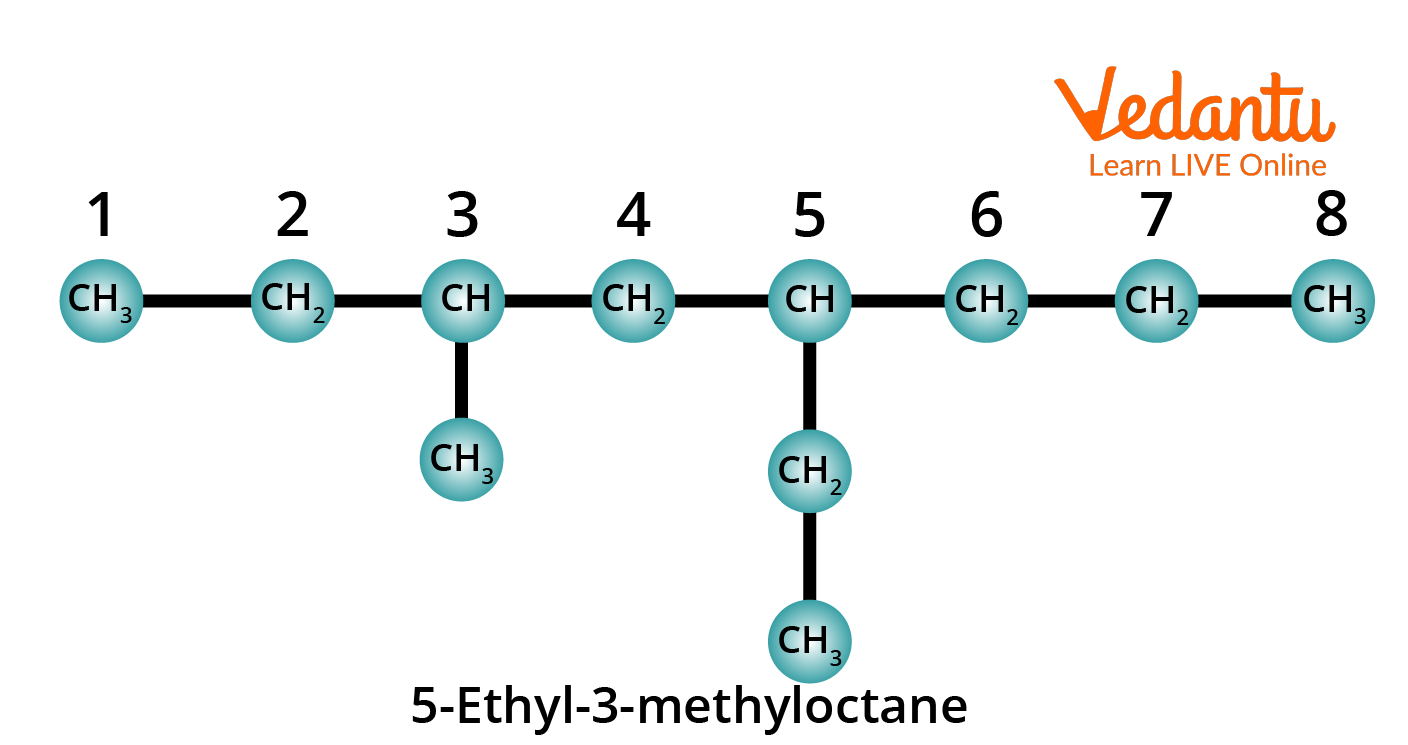
Structure of 5-Ethyl-3-Methyloctane
FAQs on Naming of Alkanes for JEE
1. What are cycloalkanes?
Cycloalkanes are alkanes in which the carbon atoms are arranged in the form of a ring. They are named by attaching the prefix cyclo- to the names of the alkanes that have the same number of carbons in the ring. Cycloalkanes are often represented by simple geometrical planar figures. Remember that Cyclopropane is represented by a triangle, Cyclobutane is represented by a square, Cyclopentane is represented by a pentagon, and Cyclohexane is represented by a hexagon. Substituted cycloalkanes are termed Alkyl Cycloalkanes.
2. Using the IUPAC Nomenclature method, name the following alkane.
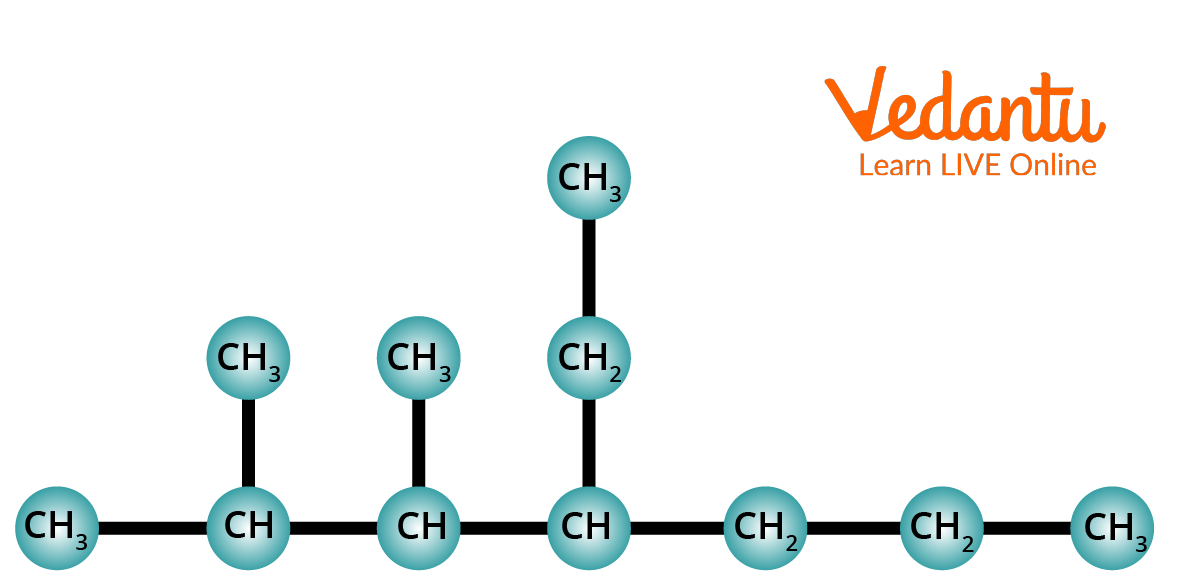
Structure of 4-Ethyl2,3dimethylheptane.
Step 1: Identify the longest chain. In this compound, the longest chain contains seven carbons. A seven-carbon chain is called Heptane.
Step 2: The chain is numbered from left to right. This gives the lowest numbers to the alkyl groups that are attached to the chain.
Step 3: Identify the alkyl groups. There are two methyl groups at C-2 and C-3. There is one ethyl group at the 4th carbon of the chain.
Step 4: Write the IUPAC Name. In this case, the IUPAC name is 4-Ethyl2,3dimethylheptane.
























