




Electrophilic Substitution of Benzene
Benzene takes part in a number of chemical reactions. One such category of reactions is electrophilic substitution reactions. In an electrophilic substitution reaction of benzene, one of its hydrogen atoms gets substituted by an electrophile. An electrophilic aromatic substitution reaction occurs in three steps, including the generation of an electrophile, followed by the attack of an electrophile, and finally deprotonation and product formation.
These reactions are highly spontaneous as the aromaticity of benzene remains undisrupted. Some of the examples of electrophilic substitution reactions are halogenation, Friedel Craft’s alkylation and acylation, nitration and sulphonation, etc. In this topic, we’ll learn about the mechanism of nitration and sulphonation of benzene.
Nitration of Benzene and Its Mechanism
Nitration of Benzene:
Nitration is the name given to the process of attaching the nitro group (-NO2) to a molecule. Both aliphatic and aromatic compounds get nitrated. Aliphatic nitration follows a free radical mechanism, while aromatic nitration follows an electrophilic substitution mechanism.
In the presence of concentrated sulphuric acid, benzene reacts with concentrated nitric acid at a temperature not exceeding $55^{\circ}C$ to form nitrobenzene. This reaction is known as the nitration of benzene. At higher temperatures, there is a possibility of more than one nitro group being substituted in the ring.
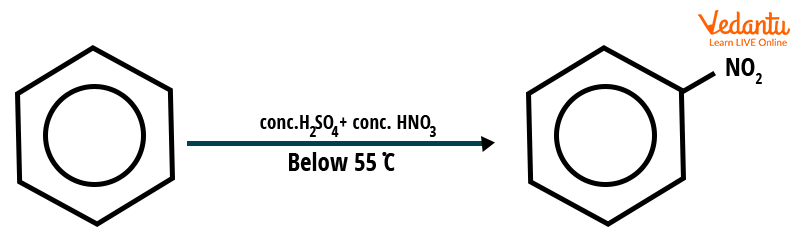
Nitration of benzene
Mechanism:
Step 1: This step is the generation of an electrophile, which here is a nitronium ion. It gets formed when nitric acid accepts a proton from sulphuric acid and then dissociates.
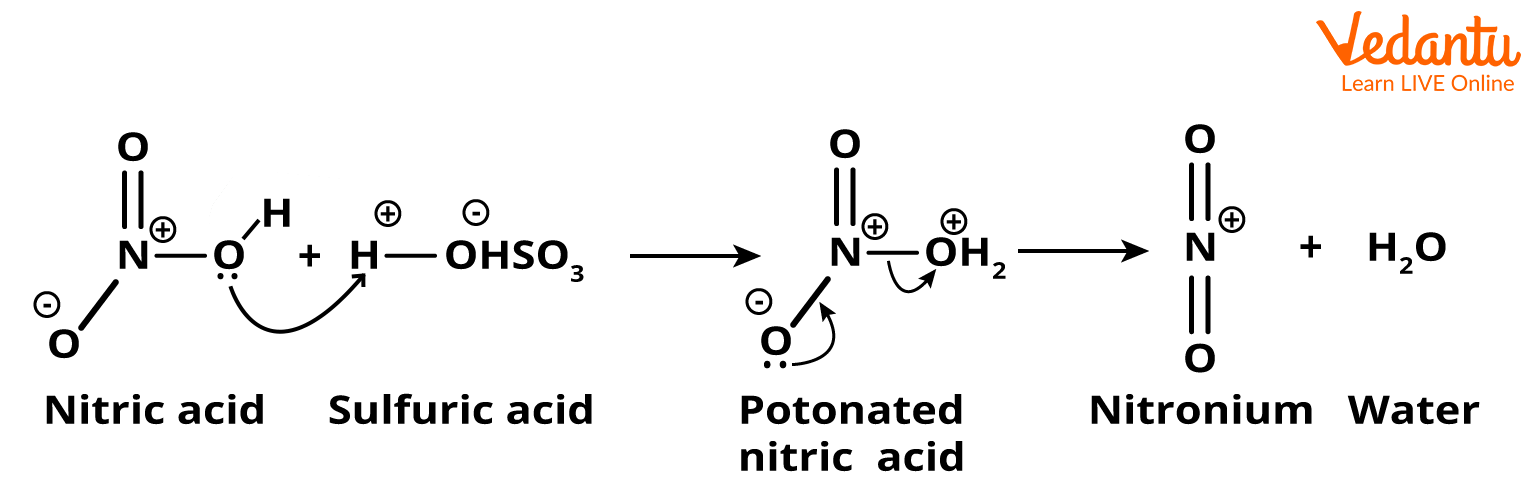
Step1-Generation of an Electrophile (Nitronium ion)
Step 2: This step is an electrophilic attack and intermediate (carbocation) formation. The nitronium ion acts as an electrophile and is attacked by the benzene ring to form an intermediate, the arenium ion, which is a carbocation, stabilised by resonance.
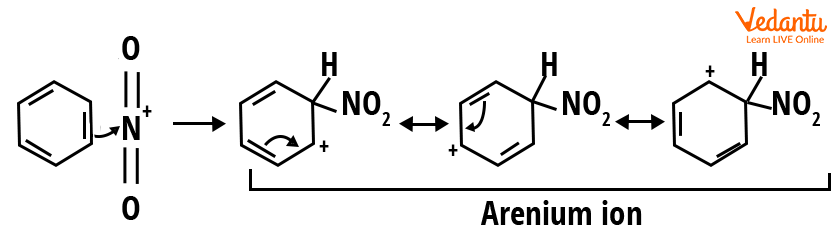
Step 2-Electrophilic attack and carbocation formation
Step 3: The third and final step is deprotonation. Arenium ion forms nitrobenzene by losing a proton to a weak base.
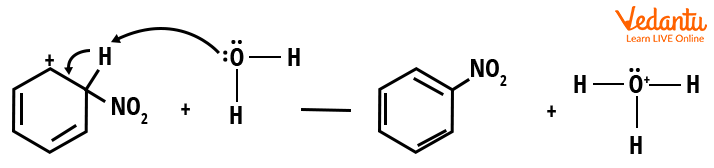
Step 3-Deprotonation and formation of nitrobenzene
Sulphonation of Benzene and its Mechanism
Sulphonation of Benzene: Sulphonation is the name given to the reaction of attaching a sulphonic group (SO3H) to a molecule. Aliphatic compounds are less reactive towards sulphonation than aromatic compounds because they are less nucleophilic in nature.
Benzene is heated with fuming sulphuric acid to produce benzenesulphonic acid. Fuming sulphuric acid is the solution of sulphur trioxide in sulphuric acid. Sulphur trioxide is electrophilic due to its highly polar nature and strong charge on sulphur. This reaction is reversible in nature.
Mechanism:
Step 1: Sulphur trioxide gets protonated through sulphuric acid and the electrophile is
generated.
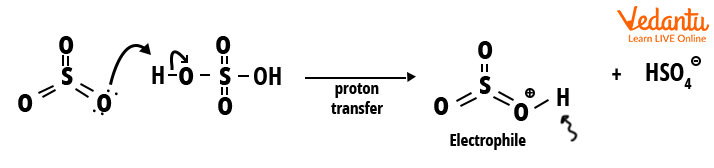
Step 1: Generation of electrophile
Step 2: Step 2 involves the attack of an electrophile and intermediate formation. The highly electrophilic SO3H is attacked by the benzene ring and a resonance stabilised carbocation is formed.
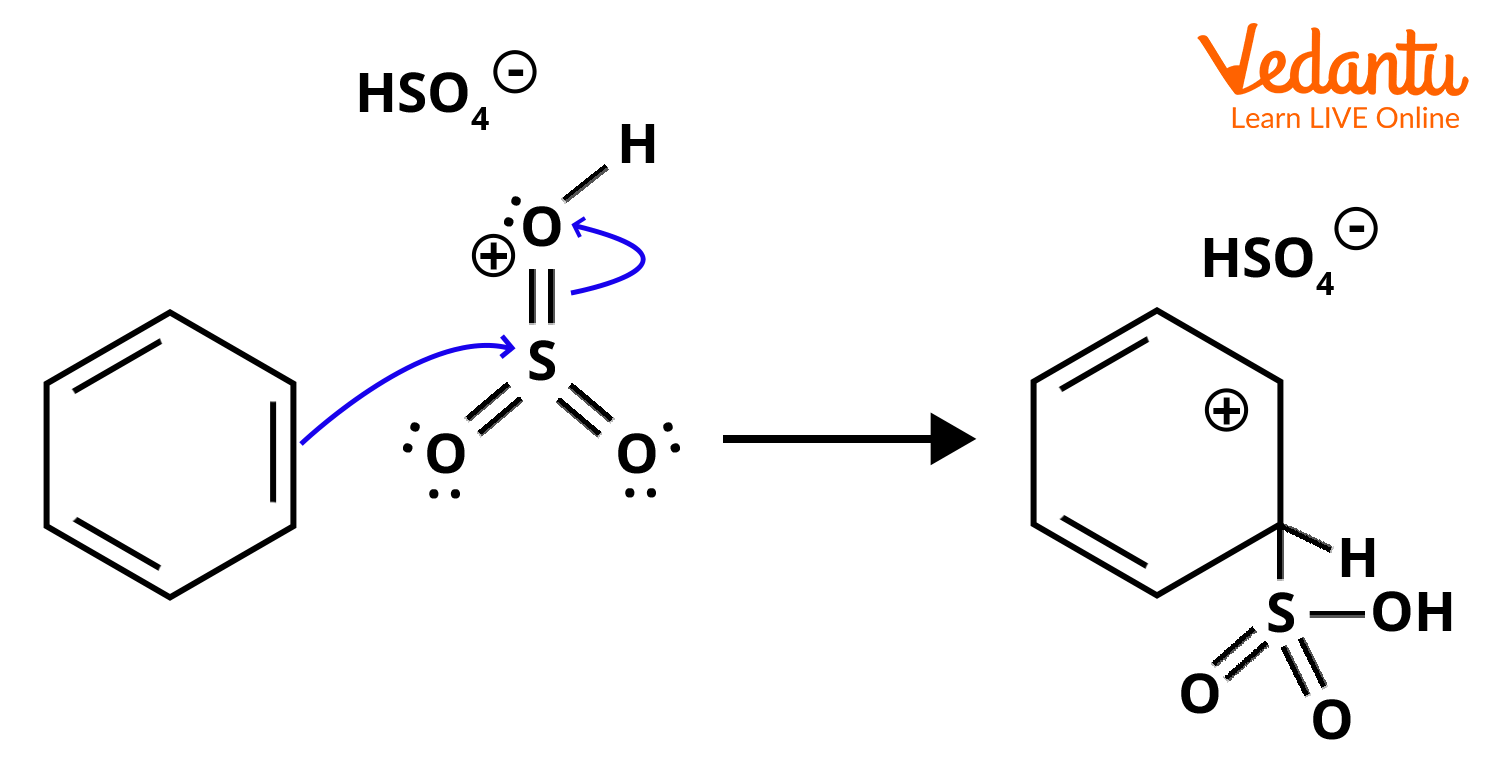
Step 2: Electrophilic attack and carbocation formation
Step 3: The final step is proton loss (deprotonation), which occurs when a weak base deprotonates the carbocation, restoring aromaticity and forming benzenesulphonic acid.
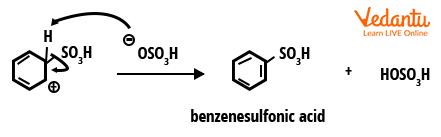
Step 3: Deprotonation and formation of products
Application of Nitration and Sulphonation
The products of aromatic nitrations are considered very important precursors and intermediates in industrial chemistry. This is because nitration is used to add nitrogen to a benzene ring, and the Nitro group deactivates the ring, which is further used extensively in substitution reactions. Nitrobenzene is an example of one such compound. Nitration reaction is also used in the production of explosives like trinitrotoluene from toluene.
Sulphonation can also be used in further substitution as the sulphonic group protects the carbon from other substituents. The sulphonation product of benzene, benzenesulphonic acid, has many useful applications in the synthesis of detergents, drugs, and dyes.
Conclusion
Both nitration and sulphonation of benzene are examples of electrophilic aromatic substitution. The whole reaction process consists of three steps. The first step involves the generation of an electrophile, which is a nitronium ion in nitration and protonated sulphur trioxide in sulphonation. In the second step, a carbocation is formed in both the reactions and the carbocation is stabilised by resonance. The third and final step is the loss of a proton and the formation of the final products. In this step, aromaticity is restored.
Both nitration and sulphonation reactions produce useful intermediates for further substitution in industrial chemistry. Nitration is extremely exothermic and can be explosive along with the production of acid vapours (nitric or sulphuric acid). Sulphonated aromatic compounds can also be pollutants in the environment.
FAQs on Nitration and Sulphonation - Their Mechanisms for JEE
1. Why is concentrated H2SO4 used in nitration?
In the procedure to produce nitrobenzene, benzene is treated with a mixture of nitric acid and concentrated sulphuric acid. This mixture is kept for 30 min at a temperature not exceeding $55^{\circ}C$. This results in the formation of yellow-coloured nitrobenzene as a product. Sulphuric acid plays an important role in this reaction as the first step of the reaction is to activate nitric acid by protonation, which produces a strong electrophile nitronium ion. Sulphuric acid is the source of proton in this step.
2. What is sulphonation used for?
Sulphonation is one of the major industrial chemical processes which is used to make a variety of products, including dyes and colour intensifiers, pigments, pesticides, medicinals, and organic intermediates.
For example, Sodium Xylenesulphonate is used in shampoos, detergents, and the textile industry, because of its potential to enhance the ability of water to dissolve other molecules. Such compounds are known as hydrotropes. It is also used in the paper industry to remove lignin and in the leather industry as a glue additive.
3. Are sulphonation and sulphation the same?
The answer is no. Although both sulphonation and sulphation are reactions that introduce sulphur-containing groups into molecules, the reactions and products are not the same. Sulphonation is used to prepare organic sulphonic acids. In this process, the organic compounds react with compounds like sulphur trioxide and sulphuric acid. Sulphation is also an important chemical process used to form sulphates as products. The key difference between the two processes is that sulphonation involves the formation of C-S bonds, while sulphation produces C-O-S bonds.
























