




How to Identify Optically Active Allenes, Spiro Compounds, and Biphenyls
Understanding Optical Isomerism in Allene, Spiro, and Biphenyl is crucial for mastering advanced stereochemistry in JEE Main. While typical optical isomerism involves chiral carbons, certain molecules without chiral centers—like allenes, spiro compounds, and biphenyls—show optical activity because of their unique three-dimensional orientation and symmetry exclusion. Quickly identifying these cases helps you solve MCQs on isomerism with speed and accuracy.
Optical Isomerism: Core Concept and Importance in JEE
Optical isomerism arises when molecules with the same molecular formula differ in their ability to rotate plane-polarized light. Such molecules, known as optical isomers, are non-superimposable mirror images (enantiomers) and lack an internal plane of symmetry. This property is critical for certain biological and material properties, and for rapidly evaluating stereochemical MCQs in JEE Chemistry.
- Optical isomers: Same structure and bonding, but differ in optical rotation.
- Cause: Lack a plane of symmetry (PS) or center of symmetry (CS).
- Enantiomers rotate light equally, in opposite directions (+/−).
- Commonly found in molecules with at least one chiral center—but also possible in specific arrangements without one.
Stereochemistry of Allenes: When Are They Optically Active?
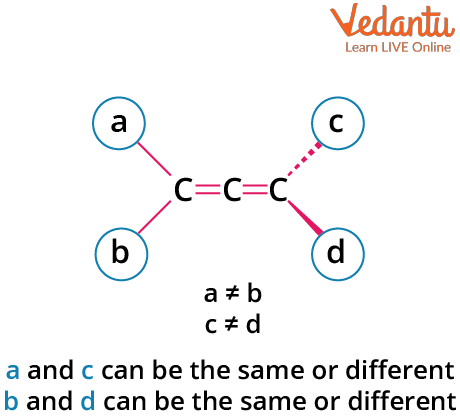
Allenes are hydrocarbons with structure C=C=C, where the central carbon is sp hybridized. The p-orbitals of the terminal carbons lie in planes perpendicular to each other, causing the attached substituents to be in distinct planes. This three-dimensional arrangement creates the possibility for optical isomerism—if paired groups on each end are different, and there is no plane of symmetry.
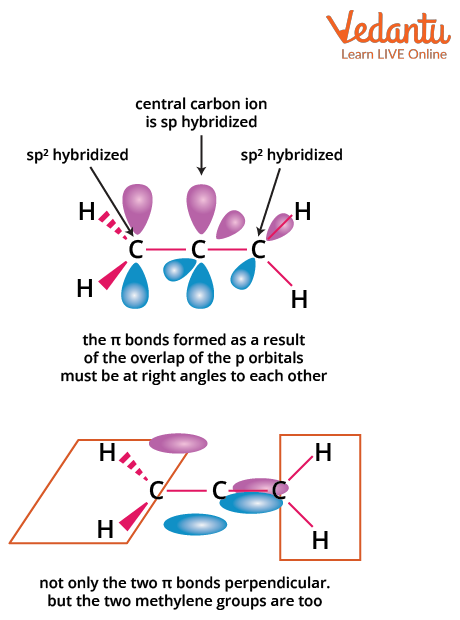
For optical activity in allenes (especially for JEE Main MCQs), remember these conditions:
- Both terminal carbons must have two different groups attached.
- No plane of symmetry or center of symmetry should exist.
- Not all allenes are optically active—check for symmetry elements each time.
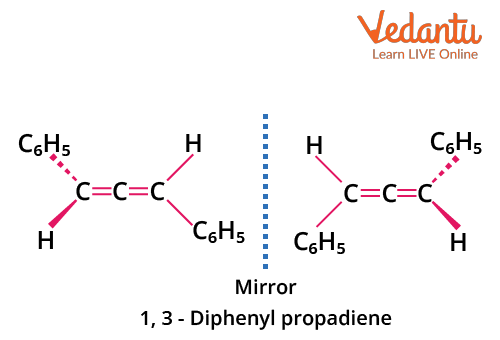
Example: 1,3-diphenylallene (PhCH=C=CHPh) is optically active if each terminal carbon bears two different groups (e.g., H and Ph). Avoid the common mistake of assuming all allenes are chiral.
Optical Isomerism in Spiro Compounds
Spiro compounds consist of two (usually non-identical) rings joined at a single central carbon. The spiro carbon is sp3 hybridized, and the orientation of the rings is perpendicular. This geometry makes spiro compounds non-planar and enables the possibility of optical isomerism if the attached groups and/or rings lack symmetry.
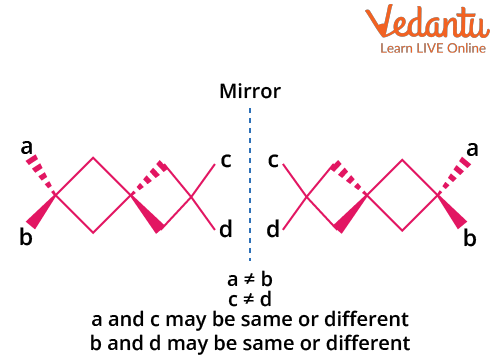
Key conditions for optical activity in spiro compounds:
- Both rings attached to the spiro carbon must be different to prevent symmetry.
- No plane of symmetry through the spiro carbon and rings.
- Non-planarity is essential—planar spiro systems do not show optical activity.
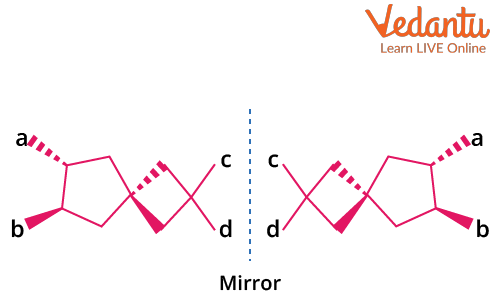
Example: Spiro[4.5]decane with different ring substituents is optically active, but if both rings are identical and symmetrically substituted, the compound is inactive.
Biphenyl Compounds: Axial Chirality and Optical Activity
Biphenyl compounds feature two aromatic rings connected by a single sigma bond. Optical isomerism arises when orthogonal (perpendicular) rings cannot rotate freely due to bulky orthosubstituents, resulting in axial chirality. Only biphenyls with suitable ortho substitution and no plane of symmetry in the overall molecule show optical activity.
- Both rings must have bulky substituents at the ortho positions that block rotation.
- Ortho substituents on one ring must differ from those on the other ring.
- The absence of a plane of symmetry (no identical substitution pattern).
- Flexible/planar biphenyls (without steric hindrance) DO NOT show optical isomerism.
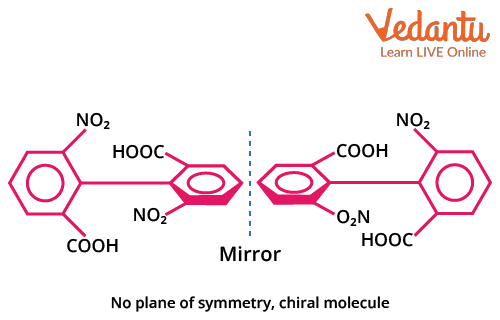
Example: 2,2',6,6'-tetra-substituted biphenyls with different groups at each ortho position are optically active. But biphenyls unsubstituted at both rings, or with flexible smaller groups, are optically inactive.
Rules for Identifying Optical Isomerism in Allene, Spiro, and Biphenyl
Use this checklist in exams to spot optically active molecules without chiral carbons:
- Check for a plane of symmetry or a center of symmetry using quick visualisation—if present, the molecule is optically inactive.
- For allenes, verify both ends have different groups attached.
- For spiro compounds, ensure the rings or substituents differ around the spiro carbon.
- For biphenyls, confirm bulky, different ortho groups on both rings, and check for restricted rotation.
- If two or more symmetry-breaking features are present, draw the mirror image and test for non-superimposability.
JEE Application Table: Fast Optical Activity Reference
| Compound Type | Condition(s) for Optical Activity | MCQ Shortcut |
|---|---|---|
| Allenes | Both terminal C atoms with two different groups; no plane/center of symmetry | Check group identity on both ends fast |
| Spiro compounds | Asymmetric rings and non-planarity; no mirror plane | Look for ring comparison, not just ring size |
| Biphenyls | Bulky ortho groups on both rings; differ across rings; restricted rotation | Draw rings at 90°; check ortho groups |
Common Mistakes and JEE Traps to Avoid
- Assuming all biphenyls or allenes are optically active—always analyze substituent identity.
- Overlooking internal symmetry in spiro or biphenyl compounds.
- Missing the effect of free rotation (especially in biphenyls with small ortho groups).
- Confusing conformational isomerism with true optical isomerism.
- Ignoring non-chiral, optically active molecules in rapid screening.
For deepening your understanding of related topics, see Optical Isomerism Concepts, Structural Isomerism, and Chiral Centre and Enantiomers on Vedantu. The Chemistry Syllabus for JEE Main provides a full view of all covered areas.
Summary Table: Optical Isomerism in Allene, Spiro, Biphenyl
| System | Chiral Centre? | Geometric Need | Active if... |
|---|---|---|---|
| Allene | No | Perpendicular terminal planes | Ends have different groups |
| Spiro | No | Non-planar, unique rings | Rings/substituents differ |
| Biphenyl | No | Restricted rotation, perpendicular rings | Bulky, different ortho groups |
Master optical isomerism in allene, spiro, and biphenyl systems for a strong advantage in JEE organic chemistry. Always check for symmetry elements, group identity, and non-planarity. For fast MCQ-solving, refer to the tables and diagrams above, and practice more using Vedantu’s expert-curated quizzes and JEE Chemistry practice papers.
FAQs on Optical Isomerism in Allenes, Spiro, and Biphenyl Compounds
1. What is optical isomerism in allenes and biphenyls?
Optical isomerism in allenes and biphenyls refers to the phenomenon where these compounds can exist as non-superimposable mirror images (optical isomers) due to their unique structural features, even without a classical chiral carbon.
Key points:
- Occurs when the molecule lacks a plane of symmetry.
- Allenes: Optically active if both terminal groups are different.
- Biphenyls: Require bulky ortho-substituents to restrict rotation and generate chirality (axial chirality).
2. What are the conditions for biphenyl compounds to show optical isomerism?
A biphenyl compound shows optical isomerism if it meets certain structural conditions that prevent free rotation and eliminate internal symmetry.
Essential conditions:
- Both phenyl rings have bulky ortho-substituents to hinder rotation.
- The molecule lacks a plane of symmetry.
- No chiral carbon is required; chirality is due to the axis between the rings (axial chirality).
- The substituents on the two rings must not be identical or symmetrically placed.
3. What are the structural requirements for optical activity in allenes?
For allenes (C=C=C compounds) to be optically active, specific structural requirements must be fulfilled:
- The terminal carbons (C1 and C3) must each have two different groups attached.
- The molecule must not have a plane of symmetry.
- No classical chiral (stereogenic) center is needed—chirality arises from the arrangement of substituents on the perpendicular planes.
- Example: 1,3-disubstituted allenes with all different substituents exhibit optical activity.
4. What is the stereochemistry of spiro compounds and when are they optically active?
Spiro compounds contain two rings joined at a single atom (the spiro center). Their optical activity depends on lack of symmetry and the nature of the groups attached to the rings.
Criteria for optical activity in spiro compounds:
- The rings are of different sizes or have different substituents.
- There is no plane of symmetry passing through the spiroatom.
- Chirality arises if all four groups around the central atom are different, similar to chiral carbons in other systems.
5. Why are some biphenyls not optically active?
Some biphenyl compounds are not optically active because they do not meet the structural requirements for axial chirality.
- If the rings can freely rotate due to lack of bulky ortho-substituents, the molecule is achiral.
- If the substituents on both rings are identical and symmetrically placed, a plane of symmetry exists.
- The presence of a symmetry element prevents optical isomerism.
6. How can I identify an optically active spiro compound in JEE questions?
To identify optically active spiro compounds in JEE exams, apply these steps:
- Check if the two rings attached at the spiro center are different or have different substituents.
- Look for absence of a plane of symmetry through the spiroatom.
- Ensure no identical groups are directly opposite across the spiro center.
7. What mistakes should students avoid when determining optical isomerism in allenes, spiro, and biphenyls?
Common mistakes in identifying optical isomerism in allenes, spiro, and biphenyls include:
- Assuming a chiral carbon is always necessary (axial/chiral center suffices in these cases).
- Ignoring planes of symmetry—always check for any mirror symmetry first.
- Forgetting about hindered rotation in biphenyls.
- Not checking all substituent groups for sameness or difference.
- Missing meso forms or internal compensation.
8. Can conformational flexibility remove optical activity in biphenyl compounds?
Yes, conformational flexibility—such as free rotation around the biphenyl bond—can destroy axial chirality, making the compound optically inactive.
- Biphenyls must have bulky ortho-substituents to restrict rotation and stabilize chiral conformations.
- If free rotation is possible (no steric hindrance), the molecule remains achiral.
9. Are all optically active molecules chiral at a central carbon?
Not all optically active molecules have a chiral central carbon.
- Allenes and biphenyls can be optically active due to axial chirality or arrangement of groups in space.
- Spiro compounds may exhibit chirality depending on structure.
- Chirality can arise from arrangements other than classical stereocenters.
10. Can meso forms exist in allenes or biphenyls?
Meso forms—molecules with stereocenters but overall symmetry—are rare but possible in specific allenes or biphenyls if symmetry elements are present.
- If substituents on terminal positions or rings are mirror images and a plane of symmetry exists, a meso form (optically inactive) can be present.
- Most exam-relevant examples focus on chiral, non-meso forms.























